Introduction
In the last decade the prevalence of urolithiasis has increased up to 10.6% in men and up to 7.1% in women [1], with especially high recurrence rates (39% at 15 years) for both genders [2]. All these changes have led to an increased number of urological procedures for stone disease patients. Thus, as technology has advanced significantly, minimally invasive procedures have gained a huge interest with the intention to reduce the treatment toxicity profile to the minimum.
Extracorporeal shockwave lithotripsy (ESWL) is commonly used to treat patients with upper urinary tract stones. Reports from high volume centers with static machines suggest stone clearance rates of 86–89%, 71–83%, 73–84% and 37–68% for calculi in the renal pelvis, upper calyx, middle calyx and lower calyx, respectively [3]. Thus, proper patient selection is one of the main cornerstones for improved ESWL efficacy and optimal disease management. Current literature suggests various predictors related to the stone and patient, which could influence stone fragmentation and clearance rates [4, 5]. Such predictors include clinical parameters, such as body mass index as well as computed tomography parameters, such as stone location, skin to stone distance, diameter or stone volume, and Hounsfield units [5, 6].
Aim
The aim of the present study was to establish clinical characteristics of stone disease for patients undergoing ESWL which may contribute to success of the procedure.
Material and methods
Patients with renal stone disease diagnosed by non-contrast computed tomography (NCCT) who underwent ESWL at a tertiary-level urology center between January 2015 and December 2019 were retrospectively included in the study. Three hundred and five cases were analyzed in total. Of these only 109 patients met study inclusion criteria with accessible NCCT scans and stone size ranging from 5 to 15 mm. The exclusion criteria were absolute contraindications to ESWL such as pregnancy, active urinary infection, uncorrected coagulopathy and patients currently anticoagulated. Patients who did not have NCCT before treatment, maximum stone diameter was over 15 mm or had multiple stones on the same side were excluded from the study. All patients were treated under the same protocol in the supine position using a Storz Modulith SLK lithotripsy machine using ultrasound guidance (Storz Medical, Germany) without anesthesia. ESWL procedures were performed by four different urologists. During the ESWL procedure up to 3 000 shock waves were delivered to the stone with a gradual power increase up to 75 mJ, maintaining frequency of 1.5 Hz during all the session. The total amount of energy applied to the stone was calculated using the Storz Medical Lithotripsy Index (SMLI). No other treatment, including internal stenting before the procedure or medical expulsion therapy afterwards, was prescribed to the patients.
Variables such as skin-to-stone distance (SSD), maximum stone length (MSL), stone volume (SV), stone surface area (SSA), mean stone density in Hounsfield units (MSD) and highest Hounsfield unit (HHU) score were obtained pre-operatively from NCCT images. SSD was calculated as the average distance from the skin to the surface of targeted stone at 0°, 45° and 90° angles on NCCT. MSL was measured in the sagittal, transversal and coronal body planes. SV was calculated using the formula: SV = l × w × d × π × 0.167, where l is length, w is width, d is depth and π = 3.14159. For the measurement of stone density, all three body planes were defined for each stone. In each plane an area of interest, smaller than the stone, was depicted where stone density was measured, and the mean value was calculated.
All patients were followed up at the outpatient clinic of the same institution 4 weeks after the treatment. Ultrasonography or NCCT was used to evaluate residual fragments. Success of the procedure was defined as the patient being stone free (SF) or when < 4 mm fragments were detected. In case of treatment failure, patients underwent repeated ESWL sessions up to 3 times within a 1-month interval between the procedures.
Statistical analysis
Continuous variables are presented as means with standard deviations (SD). Data for categorical variables are presented as frequencies and percentages. Continuous variables were checked for normal distribution by the Shapiro-Wilk test and compared by the t-test when normally distributed or the Mann-Whitney U test for non-normally distributed variables. Pearson’s χ2 and Fisher exact tests were used for comparison of categorical variables, as appropriate. To identify predictors for ESWL failure, univariate and multivariate logistic regression analysis was performed, where odds ratios (OR) and 95% confidence intervals (CI) were calculated. Receiver operating characteristic (ROC) curves were generated and areas under the curves (AUC) were calculated to compare the predictive power of different characteristics. All statistical tests were performed using SPSS software (IBM Corp., Armonk, NY, USA). P-value of < 0.05 was considered as statistically significant.
Results
Overall, 109 patients were included in the study. Baseline demographic and clinical characteristics of the study cohort are summarized in Table I.
Table I
Baseline demographic and clinical characteristics of the study cohort
| Variable | All patients (N = 109) | Successful treatment (N = 73) | Treatment failure (N = 36) | P-value* |
|---|---|---|---|---|
| Age [years] Mean ± SD | 54.3 (14.7) | 55.8 (13.8) | 51.2 (16.7) | 0.13 |
| Gender, n (%): | ||||
| Female | 46 (42.2) | 32 (43.8) | 14 (38.9) | 0.68 |
| Male | 63 (57.8) | 41 (56.2) | 22 (61.1) | |
| Stone location, n (%): | ||||
| Lower calyx | 54 (49.5) | 37 (50.7) | 17 (47.2) | 0.83 |
| Other | 55 (50.5) | 36 (49.3) | 19 (52.8) | |
| Max. stone diameter [mm] Mean (± SD) | 10.0 (3.6) | 8.3 (2.6) | 10.8 (3.7) | 0.01 |
| Stone volume [mm3] Mean (± SD) | 269.5 (232.7) | 150.8 (123.3) | 328.0 (251.6) | 0.01 |
| Stone surface area [mm2] Mean (± SD) | 54.3 (35,1) | 36.8 (23.0) | 62.8 (36.9) | 0.02 |
| Stone attenuation value [HU]: | ||||
| Maximum (± SD) | 1170.9 (375.6) | 1092.4 (422.6) | 1209.7 (346.6) | 0.12 |
| Mean stone density (± SD) | 804.2 (282.1) | 762.1 (301.0) | 824.9 (271.9) | 0.27 |
| Skin to stone distance [mm] Mean (± SD) | 110.2 (24.3) | 104.4 (20.8) | 113.0 (25.5) | 0.03 |
| SMLI Mean (± SD) | 167.3 (33.1) | 167.4 (32.4) | 167.3 (35.1) | 0.98 |
| SMLI/stone volume ratio Mean (± SD) | 1.3 (1.5) | 2.0 (1.8) | 1.0 (1.1) | 0.01 |
Stone size reduction was observed in 77 out of 109 (70.6%) patients, while success of the procedure was achieved in 73 (67.0%) patients. Treatment failure was associated with larger stones, greater distance to the target and less energy applied to the stone volume unit during the procedure (Table I, all p < 0.05).
During univariate logistic regression analysis stone size outperformed other clinical characteristics and revealed the highest prognostic power for ESWL failure, where OR for stone volume and stone surface area were 1.06 (1.03–1.10) and 1.04 (1.02–1.06), respectively (Table II, all p < 0.01) while a tendency was observed for skin to stone distance 1.02 (1.00–1.03). Amount of energy applied during the procedure to one cubic millimeter of stone volume (SMLI/stone volume) was predictive for treatment success (OR = 0.60, 95% CI: 0.41–0.87, p < 0.01). In multivariate logistic regression analysis (Table II), stone volume (OR = 1.06, 95% CI: 1.00–1.14, p = 0.01) and stone surface area (OR = 1.03, 95% CI: 1.01–1.06, p = 0.02) remained as statistically significant prognostic factors for treatment failure.
Table II
Univariate and multivariate logistic regression analysis of the associations between clinical characteristics and treatment failure
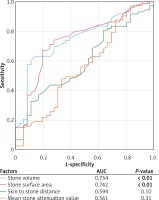
In ROC analysis (Figure 1), stone volume was the most significant independent predictor for ESWL failure, where AUC for stone volume was 0.754 (p < 0.01) and AUC for stone surface area was 0.742 (p < 0.01), while the same tendency was observed for skin to stone distance (AUC = 0.594, p = 0.10).
Discussion
The ESWL procedure provides good treatment outcomes [6] with similar overall complication rates as for endourological procedures, such as retrograde ureteroscopy [6, 7]. However, many factors, associated with stone characteristics and constitution of the patient, as well as technical aspects of ESWL, have been identified to influence treatment efficacy [4, 5].
According to the literature, stone-free status after ESWL monotherapy for stones of size < 20 mm is 80–85% [8, 9], as compared to 33–65% for stones > 20 mm [5, 10]. Taking all these findings into consideration, ESWL is recommended for kidney calculi smaller than 20 mm [11]. These findings are in line with our results, where stone size was significantly associated with treatment outcomes and greater stone volume was detected in patients with ESWL failure.
Lower pole renal calculi provide a unique challenge when considering the ESWL procedure due to a lower stone clearance rate – 52–69% [12–14]. In our study a considerably high number of patients (49.5%) underwent ESWL due to calculi in the inferior calyx, while no significant differences were observed in primary treatment outcomes. All these findings confirm the idea that stone disintegration efficacy of the ESWL procedure is the same in any part of the kidney, while stone clearance from the lower part of the kidney could be affected by the infundibular angle or a narrow calyx.
Increased BMI could be an independent predictor for ESWL outcomes. Pareek et al. evaluated patients who underwent ESWL for a solitary renal stone measuring between 5 and 10 mm using an electrohydraulic lithotripter. After follow-up 72% were stone free and 28% had residual fragments larger than 3 mm. The stone-free patients had a significantly smaller mean BMI (26.9 ±0.5 kg/m2) than patients with residual fragments (30.8 ±0.9). Logistic regression analysis performed by the authors revealed that an unsuccessful outcome was statistically significantly related to the BMI (OR = 1.34, p < 0.01) [15]. In a larger cohort of 688 patients, Delakas et al. using a second-generation electrohydraulic shockwave lithotripter found no association between increased BMI and ESWL failure [16]. However, more studies suggest that high BMI affects outcomes of the ESWL. Garrido-Abdad et al. also reported that high BMI was one of the parameters that showed a significant difference after multivariate analysis. The cut-off value was 26.9 kg/m2 [17].
Skin-to-stone distance (SSD) is mostly calculated as the mean of the distances from the body surface to a targeted stone at 0°, 45°, and 90° angles on NCCT using radiographic calipers. BMI is an important parameter in predicting ESWL success. Some authors have concluded that the SSD value is a more predictive parameter than BMI due to different body types and body fat distribution among people and races. Pareek et al. revealed that SSD (using a cutoff value of 10 mm) was a much more powerful predictor than BMI and hypothesized that the travelling of shock waves for longer distances is associated with attenuation of those shock waves [4]. Similarly, Wiesenthal et al. reported that the cutoff value for SSD was 11 mm [18]. It has been noted in previous studies that failure of ESWL is related to greater SSD. The mean SSD for ESWL success was 8.12 ±1.74 cm against 11.53 ±1.89 cm in the ESWL failure group (p < 0.01) [15]. According to another study, the mean SSD in the stone-free group was 83.3 ±21.9 mm compared to the residual stone group where the mean SSD was 107.7 ±28.9 mm (p < 0.050) and SSD was the only significant independent predictor of treatment outcome [19]. A success rate of 71.4% was noted in patients having SSD less than 100 mm in contrast to 46.2% in those with SSD greater than 100 mm, showing the OR of 1.036 (95% CI: 1.014–1.059; p < 0.01). Moreover, SSD was noted to be 90.65 mm in the success group compared with 104.33 mm in the failure group, which favors the suggested cut-off value [13]. Shinde et al. reported that in their study the success group had a mean SSD of 103.9 ±21.3 mm while the mean SSD in the failure group was 111.6 ±22.4 mm [20]. Our analysis results are in line with other authors, where the successful treatment group had a mean SSD of 104.4 ±20.8 mm and the failure group 113.0 ±25.5 mm (p < 0.01).
However, there are some studies in Asian populations with contradictory results. Because Asian populations have thin bodies compared to Western populations, it was argued that it could not be applied to Asian patients. Various studies reported that SSD was not a meaningful factor [21].
Stone attenuation value (SAV) is mostly measured by creating three regions of interest in three different views of the stone on the NCCT scan showing the stone in the largest dimension. It is presented by the mean values of defined stone regions in Hounsfield unit (HU) [13]. Other authors calculated SAV using mean attenuation of three consistent (area 0.02 cm2), non-overlapping regions of interest chosen from stones in bone windows [21].
There is a conclusion that stones with higher density require a larger amount of shock waves [15, 21, 22]. Furthermore, in 2013, Hameed et al. [23] reported similar results and concluded that stones having > 1,350 HU require increased shock wave energy.
Nowadays most authors use a 815–1000 HU cut-off value for predicting successful ESWL. Shinde et al. observed a stone-free rate of 56.2% in patients with stones > 1000 HU density compared to that of 87.7% with a stone density ≤ 1000 HU [20]. In Wiesenthal’s study and another by Wang, stones with > 900 HU were more likely to fail after ESWL [18, 24]. Nakasato et al. reported that success rates following ESWL were significantly higher for stones < 815 HU than for stones > 815 HU (p < 0.02) [25]. In another study, Quzaid et al. reported that stone density of 970 HU was the most sensitive point in the determination of stone density. They achieved 96% stone-free rates for stones < 970 HU and 38% stone-free rates for stones ≥ 970 HU (p < 0.001) [26].
Patients having SAV less than 500 HU were much more likely to have stone clearance (93.8%), while those having values greater than 1,000 HU were much less likely to experience successful outcomes (24.5%), even with an increasing number of shock waves [13]. Other authors also published perfect results for patients with stones < 500 HU [14]. In our study mean stone density was not significantly different between successful ESWL and failure groups, but patients with mean stone density lower than 500 HU had successful treatment outcome in 71.1% of cases while patients with higher than 500 HU had a positive outcome only in 47.4% of cases (p = 0.06).
With the frequent use of NCCT to evaluate stone disease, stone burden is commonly measured using the axial stone diameter. The size of the stone is typically assessed by measuring the maximum length. However, kidney stones are irregular 3D structures and can have complex geometric shapes. The stone surface area is a 2D measurement of stone burden, as it takes into account the overall shape of the stone. Both stone length and surface area measured by traditional radiography fail to provide any volumetric information, as they are limited by the inability to measure the third dimension (depth of the stone). Importantly, the shape and depth of the stone might have significant implications for the total stone burden. The total volume of a cylinder is twice the volume of a sphere of the same diameter and height. These differences might be even more important when measuring the stone burden in patients with irregularly shaped stones [27].
Bandi et al. analyzed how stone volume predicts outcomes of ESWL. The mean stone volume was significantly different between stone-free and residual fragment groups (274 vs. 464 µl) and the stone volume was the strongest predictor of stone-free status. A stone volume of < 500 μl best predicted treatment success with 72% of patients with a stone volume of < 500 μl having a successful outcome vs. only 27% with a stone volume of > 500 μl [27]. Similarly, El-Nahas claimed SV as a predictive factor for disintegration of stones following ESWL [28].
Our successfully treated patient group had a mean stone volume of 150.8 ±123.3 mm3 and the failure group 328.0 ±251.6 mm3 (p < 0.01). During univariate logistic regression analysis stone size showed the highest prognostic power for ESWL failure, where ORs for stone volume and stone surface area were 1.06 (1.03–1.10) and 1.04 (1.02–1.06). In multivariate logistic regression analysis including all significant covariates, both stone volume (OR = 1.06, 95% CI: 1.00–1.14, p = 0.01) and stone surface area (OR = 1.03, 95% CI: 1.01–1.06, p = 0.02) were shown as prognostic factors for treatment failure.
A study of Tran et al. presented a simple metric for stone volume and called it ellipsoid stone volume (ESV). This metric was developed for the purpose of overcoming difficulties calculating stone volume. ESV can be rapidly determined with measurement of the anteroposterior, horizontal, and craniocaudal stone diameters. Notably, when ESV is compared with the computer-generated 3D stone volume, the correlation coefficient is 0.9893. The authors found that ESV is a strong predictor of ESWL success, with an AUC of 0.775 [29]. Waqas et al. noted a correlation between the stone burden (in terms of stone size or diameter, stone area, and stone volume) and the success rate. Stone volume (mm3) in the success group was 515.44 ±628.05 and 1,118.31 ±1,335.74 in the failure group. However, this again failed to show any significance in the multivariate analysis. This may be attributable to the homogeneity of stone size within their study population resulting in a type I error [13].
Application of optimal shock wave rates can both improve stone fragmentation and reduce surrounding tissue damage [30]. Kang et al. in a systematic review and network meta-analysis compared low (1 Hz), intermediate (1.5 Hz) and high (2 Hz) lithotripsy rates. Thirteen RCTs were included, showing that success rates of low (OR = 2.2) or intermediate frequency ESWL (OR = 2.5) were greater than for high-frequency ESWL. There was no significant difference in ESWL success rate for low and intermediate frequency ESWL [31].
A study from Hong Kong assessed the effects of a ramping protocol in patients undergoing extracorporeal shock wave lithotripsy of renal stones. They randomized patients into two groups: group 1 (first 1,000 shocks at energy level 5 followed by 1,000 shocks at energy level 6 and 1,000 final shocks at energy level 7) and a fixed voltage protocol in group 2 (all 3,000 shocks at energy level 7). Group 1 received 14.8% lower energy than group 2, which was significant (p < 0.001). The treatment success rate in groups 1 and 2 was 67.8% and 73.6%, respectively, which was not significant (group 1 crude OR = 0.753, 95% CI: 0.456–1.244, p = 0.268). The difference in stone-free rates in groups 1 and 2 was 36.6% and 41.9% and was not significant as well. However, there was a significant difference in perinephric hematoma development rates in group 1 and 2, observed in 23.8% and 43.8% of patients respectively (p < 0.001) [32]. In our study we used 90 shocks per minute during ESWL procedures. Power ramping also was used. Shock wave power was gradually increased up to 75 mJ. We did not find any current studies analyzing how power delivered per stone volume unit affects ESWL efficiency. During our analysis, power delivered to the stone was calculated using the SMLI index (shock waves power adjusted by shock waves rate). Successfully treated patients had a mean SMLI/stone volume ratio of 2.0 ±1.8 while the failure group had a lower ratio of 1.0 ±1.1 (p = 0.01). These results show that power delivered for a single unit of stone volume is an important factor for ESWL outcome. SMLI/stone volume was a predictive factor for treatment success (OR = 0.60, 95% CI: 0.41–0.87, p < 0.01). These findings definitely require further investigation and the optimal cut-off value is still unknown.
The impact of patient’s age on ESWL outcomes is debatable. Many studies have discussed factors affecting the outcome of ESWL, but only a few have considered age of any significance. One study of 3023 patients with renal and ureteric calculi treated with ESWL revealed that a significantly lower stone-free rate was associated with older age [5]. Another multivariate analysis of 2954 patients with kidney stones treated with ESWL revealed that patients aged > 40 years had a significantly poorer stone-free rate [33]. The reason for this finding is still unknown. Age-related sclerotic kidney changes may affect the acoustic impedance and lower efficacy of ESWL. Another factor could be reduced physical activity. Further studies are needed to analyze age as a predictor for ESWL outcome.
Although still under investigation, clinical nomograms have been used to guide physicians in selecting the safest and most effective treatment for specific patients. Some authors have demonstrated that when information from nomograms was used to select patients to undergo ESWL, success rates were higher [34]. Although in practice these nomograms may be complex and confusing [4, 5], recently Tran et al. reported a novel and simple nomogram, called the “Triple D scoring system”. Using computed tomography imaging, the Triple D describes three parameters: stone density, stone volume (SV), and skin-to-stone distance (SSD). The authors concluded that this scoring system may increase the success rates of ESWL by indicating appropriate patients for the treatment. The Triple D score integrates three powerful predictors of ESWL success into a single score, where a score of 0, 1, 2, and 3 correlates with success rates of 21.4%, 41.3%, 78.7%, and 96.1%, respectively [34]. Some authors criticize this system due to a lack of external validation and short postoperative follow-up period, which may underestimate the stone-free rates [33]. Ozgor et al. completed a study to externally validate the Triple D score. The conclusion was that Triple D scores were significantly higher in patients successfully treated with ESWL compared with patients in whom ESWL failed (p < 0.001). Triple D scores of 0, 1, 2, and 3 correlated with stone-free rates of 41.7%, 33.7%, 69.4%, and 97%, respectively. The multivariate analyses revealed that Triple D score and stone location were independent factors affecting ESWL success [33].
We understand that we are prevented by the sample size and retrospective nature of the study from drawing strong conclusions. Moreover, according to our practice we have evaluated outcomes 4 weeks after the procedure. Therefore, we could expect an additional fragment passing during the period of 3 months. This could lead to an overestimation of our failure rates. However, our data could be used to identify the patients who could have an early benefit from ESWL treatment in order to give the objective information to the patient before choosing an appropriate personalized treatment method.
From our point of view predictive factors taken separately cannot identify all patients who are likely to benefit from ESWL and exclude those that will have an unfavorable outcome. A modern approach should be used combining various factors, including stone location, size, skin-stone distance, BMI, and stone density. Delivered power and stone volume ratio could be useful tools to calculate the required power to fragment the stone. Specialized and verified nomograms may help to improve patient selection for ESWL procedures and further investigation is needed.
Conclusions
Both greater stone volume and stone surface area, as well as lower power delivered to the stone volume unit during the ESWL procedure could be useful to predict treatment failure. The most important predictive factor of treatment failure is stone volume. In our opinion, power delivered to the stone (SMLI) and stone volume ratio could be useful tools for ESWL procedure planning. This information can facilitate better procedure planning and patient information. Further larger prospective studies are required to verify our results.