Dry powder inhalers: similarities, differences, classifications
The purpose of this paper is to discuss systems providing feedback from the inhaler to the patient to inform about the inhalation performed with dry powder inhalers (DPIs). For this aim, publications in the PubMed database as well as the summary of product characteristics (SmPC) and information for patients regarding the most commonly used multi-dose DPIs were reviewed. Modern technical solutions related to this issue were presented in detail based on the example of NEXThaler®.
Optimal inhalation therapy for chronic inflammatory diseases affecting the lower airways, such as asthma or chronic obstructive pulmonary disease (COPD), involves delivering the highest possible dose of inhaled drug to the desired area of the airways with the lowest possible systemic exposure to the drug. It has been known for many years that the effectiveness of aerosol therapy depends on the inhaler, the drug and the patient, and the reciprocal nature of these elements affects the expected clinical effect [1].
DPIs represent a dominant group of inhalation devices that are used in the treatment of asthma or COPD [2, 3]. Several to a dozen different types of DPIs are available in various countries. These inhalers differ in a number of features such as design, internal resistance, method of powder aerosolisation, minimum and optimum inspiratory flow, characteristics of the aerosol cloud formed, method of use or feedback on the inhalation procedure to the patient [4, 5] (Table 1).
Table 1
Classification of DPIs according to the method of preparation and dispensing the drug dose (inhalers in alphabetical order) [4, 5, 12]
| Single-dose inhaler I generation | Multi-dose blister inhaler IIa generation | Multi-dose reservoir inhaler IIb generation |
|---|---|---|
| Breezhaler® | AirMaster® | Easyhaler® |
| New Generation Cyklohaler | Diskus®a | Genuair®b |
| Elpenhaler® | Ellipta® | NEXThaler® |
| Fantasmino® | Forspiro® | Novolizer® |
| HandiHaler® | Spiromax® | |
| Zonda® | Turbuhaler® | |
| Twisthaler® |
Almost all DPIs available on the market are so-called passive inhalers. These devices are entirely patient-dependent in terms of the aerosolisation process of the powder and its deposition in the airways [6]. Consideration should be given to the physical aspects of particular inhalers, such as internal resistance or optimal inspiratory flow, which directly affect the efficiency of therapeutic aerosol formation, drug deposition in the lungs, and ultimately, clinical efficacy [5, 7]. The other group consists of so-called active inhalers. These devices use external energy to aerosolise powder, e.g. compressed air, electric vibrations or mechanical rotors, and thus eliminate the necessity for the patient to generate large inspiratory flows [7–9].
From the point of view of the efficiency of aerosol therapy by means of DPI, the most important parameter is the patient’s ability to generate adequate inspiratory pressure (often assessed indirectly by measuring the peak inspiratory flow – PIF). Assessing the patient’s ability to generate a minimum pressure drop (of approximately 1 kPa) at any resistance within the range that is representative of a given DPI should be an effective way to decide whether the patient is an appropriate candidate for effective use of that DPI [10]. In practice, only the PIF is usually measured, e.g. with an In-Check device, which allows an appropriate inhaler to be selected in accordance with the patient’s inspiratory capabilities [11].
DPIs are also differentiated by the way in which an inhaler is prepped for the inhalation onset, i.e. dispensing the dose of drug (Table 1). This consideration is particularly important from the patient’s point of view. This classification divides DPIs into three major groups [4, 5, 12]:
single-dose inhalers (I Generation DPIs) – a single dose of medicine is dispensed and pre-packaged in a capsule or a blister (e.g. Breezhaler®, New Generation Cyklohaler, Elpenhaler®, Fantasmino®, HandiHaler®, Zonda®);
multi-dose blister inhalers (IIa Generation DPIs) – pre-filled blister packs containing the drug (e.g. AirMaster®, Diskus®, Ellipta®, Forspiro®);
multi-dose reservoir inhalers (IIb Generation DPIs) – containing a reservoir of medication, the dose is dispensed via a suitable onset of the inhaler prior to inhalation itself (e.g. Easyhaler®, Genuair®, NEXThaler®, Novolizer®, Spiromax®, Turbuhaler®, Twisthaler®).
Feedback systems in multi-dose dry powder inhalers
The information on the correct preparation of an inhaler to carry out the inhalation and then on the correctness of its performance that a patient can obtain is another key element related to DPI application. The proper sequence of steps to prepare the inhaler and the performance of the correct inhalation onset determine the dose of the drug, powder dispersion and aerosolisation. Although DPIs generate relatively fewer errors while being used than metered dose inhalers or nebulizers, a large proportion of patients (13–15%) still make various types of errors, including the so-called critical errors [13–15].
The risk of errors resulting in incorrect inhalation and adverse effects on the course of asthma or COPD is present at each step of the DPI inhalation process [16–19]. Therefore, the inhaler design, which prevents or reduces the risk of error in its use, and properly provided patient training on a given DPI are very important [1, 20]. The fewer actions (steps) to perform and the simpler the instructions, the easier the subsequent steps are to remember and repeat, and the lower the likelihood of error.
When preparing the inhaler for use, it is necessary to carry out one (some of IIb Generation DPIs) up to five (I Generation DPIs) consecutive steps, which may be material to the risk of error and the patient’s acceptance of the therapy [21, 22]. This risk may be reduced by selecting an inhaler with the fewest number of steps required to take a dose. Table 2 summarises some features of DPIs that are important from the user’s point of view and affecting the risk of an incorrect inhalation [5, 9, 23–29].
Table 2
| Inhaler | Number of steps during the use of the inhaler | Feedback about correct inhalation | Protection against using an empty inhaler by changing the colour of the counter or other mechanism | Protection against dose accumulation in the inhalation chamber | Protection against dose loss due to patient’s failure to receive a dose |
|---|---|---|---|---|---|
| AirMaster® | 3 | – | + | – | – |
| Ellipta® | 3 | – | + | – | – |
| NEXThaler®a | 3 | + | + | + | + |
| Spiromax®b | 3 | – | + | + | – |
| Twisthaler®c | 3 | – | – | – | – |
| Diskus® | 4 | – | + | – | – |
| Easyhaler® | 4 | – | + | – | – |
| Genuair® | 4 | + | – | – | – |
| Novolizer® | 4 | + | – | – | – |
| Turbuhaler® | 4 | – | + | – | – |
| Forspiro® | 5 | – | + | – | – |
a Opening and closing the mouthpiece without inhaling is not recorded by the dose indicator; the dose is only dispensed on the dose counter when an effective inhalation is completed. It is not possible to accidentally take a double dose during one inhalation.
b Opening and closing the mouthpiece without inhaling is recorded by the dose counter. The dispensed dose is kept in the inhaler until the next inhalation is needed. It is not possible to accidentally take a double dose during one inhalation [30].
c The click when closing the mouthpiece cap indicates that the next dose has been loaded and the inhaler is ready for use [31].
As shown in Table 2, DPIs vary considerably regarding features that are important from the user’s viewpoint [30, 31]. Only 3 of 11 inhalers provide feedback to the patient on correct completion of the inhalation (Genuair®, NEXThaler®, Novolizer®). Most of the DPIs (8 out of 11) have a safety device to prevent the use of an empty inhaler, mainly in the form of changing the colour of the dose counter window. 2 of 11 DPIs (NEXThaler® and Spiromax®) provide protection against dose accumulation in the inhalation chamber. Only one of the inhalers reviewed (NEXThaler®) includes protection against losing a measured dose that has not been taken by the patient. In the case of Spiromax® inhaler, a measured dose of medicine not taken by the patient is stored in the inhaler until the patient needs to take another dose. However, the dose is dispensed on the counter of the inhaler, so from the patient’s and payer’s point of view, the dose is lost.
There are several means (mechanisms) to provide feedback to the patient on the use of the inhaler and the correctness of the inhalation administered. In this respect, 4 levels of technical refinement of currently used multi-dose DPIs can be distinguished (Table 3) [32–39]. Table 4 summarises the feedback systems in multi-dose DPIs [33, 35, 36, 40–42].
Table 3
Technical advancement levels of the mechanisms providing feedback to the patient on the use of the inhaler and the correctness of the inhalation carried out in currently used multi-dose DPIs [32–39]
Table 4
| Inhaler | Counter type | Signal indicating the dose dispensed | Signal to confirm inhalation | System indicating a correct inhalation (inspiratory flow ≥ minimum value) | Possible digital DPI | |||
|---|---|---|---|---|---|---|---|---|
| Visual | Auditory | Visual | Auditory | Gustatory | ||||
| AirMaster® | U | – | + | – | – | + | – | – |
| Diskus®a | U | + | + | – | – | + | – | + |
| Easyhaler® | D | – | + | – | – | + | – | – |
| Ellipta® | U | + | + | – | – | + | – | + |
| Genuair® | Db | +c | + | +e | + | + | +g | – |
| Forspiro® | U | +d | + | – | – | + | – | – |
| NEXThaler® | Ub | – | + | +f | + | + | +h | + |
| Novolizer® | Db | +c | + | +e | + | + | +g | – |
| Spiromax® | U | + | + | – | – | + | – | + |
| Turbuhaler® | D | – | + | – | – | – | – | + |
| Twisthaler® | U | + | + | – | – | + | – | – |
Most of the DPIs in question (64%) are equipped with a unit dose counter whereas some others are only equipped with a decimal counter (less accurate). The auditory information that a single dose has been dispensed and that the inhaler is ready for use is provided by all the DPIs analysed, most of them (7/11) also provide visual information [23, 40, 43]. All except Turbuhaler® indicate inhalation completion through the sensation of a sweet taste resulting from the lactose carrier. In Turbuhaler® DPI, the carrier is also lactose, but due to its small amount in a single dose (< 1 mg) the patient does not experience a sweet taste [37]. Genuair®, Novolizer® and NEXThaler® additionally carry visual and auditory information about inhalation completion. These three inhalers are equipped with a system that indicates correct inhalation (inspiratory flow ≥ minimum value). For Genuair® and Novolizer® inhalers, an audible signal (click) and a change in colour of the control window (from green to red) are included, while NEXThaler® inhaler provides an audible signal (click) and a countdown on the dose counter after closing the mouthpiece only if the inhalation is correct [23, 28, 40].
The digitisation of DPIs has been taking place over the last few years. Several technological solutions have been developed, some of which are already available on the pharmaceutical market [33, 39]. There are basic digital DPIs (recording DPI use only) and advanced digital DPIs (additionally measuring PIF, time to reach PIF, inspiratory volume or duration of inspiration). High-tech digital DPIs allow to monitor correctness of the entire inhalation process in real time and to save it. Digihaler®, already available on the US market, is a very good example in this case [44]. Moreover, the data stored in the mobile application can be shared with the physician upon the patient’s consent. Such solutions have been proven to increase the number of days free of asthma symptoms, reduce the use of rescue medication and reduce the number of exacerbations [45–47].
Feedback system on the example of NEXThaler® dry powder inhaler
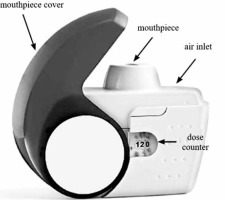
The latest feedback systems available only in some DPIs will be discussed in detail following the example of the NEXThaler® DPI (Figure 1). This inhaler incorporates several unique, original technical solutions to facilitate control of the entire inhalation process. The most important of these are: the breath-actuated mechanism (BAM) system and a system ensuring dose repeatability. The BAM system is the mechanical unit of this inhaler and its role is to delay drug delivery until the patient’s inspiratory flow rate is adequate (> 35 l/min) to detach drug particles from the lactose carrier [48]. Based on the study by Farkas et al., it is known that the BAM system leads to: a reduction in the emission of large particles > 5 μm, an increase in the available therapeutic fine particle fraction (FPF) and the deposition of the drug dose mainly in the lower respiratory tract [48]. On the other hand, a system providing dose repetition allows the full collection of a consistent dose of drug during inspiration, regardless of the inspiratory flow rate (in the range of 35–90 l/min) [28, 49].
NEXThaler® is equipped with 3 other inhalation feedback systems [28]:
a click, which is heard at the moment when the inspiratory flow exceeds 35 l/min (proof of the BAM system activation, which virtually means that the inhaler incorporates an inhalation indicator);
a unit dose counter indicating the number of remaining doses in the inhaler, which activates (slides) when the inhalation is carried out properly; and
a lactose carrier whose taste confirms that the inhalation has been completed.
Furthermore, particularly important or unique features of this inhaler include:
protection against taking multiple doses of medicine – the patient can only take 1 dose of medicine at a time;
protection against dose loss – the dose of the medicine is always returned to the reservoir if the inhalation is not carried out correctly (no dose loss);
the counter works in conjunction with inhalation – the counter does not count down the dose if the patient misses a dose;
generation of extra-fine particles with 1.1–1.5 μm Mass Median Aerodynamic Diameter, which is essential for the drug to reach the fine airways, improving pulmonary drug deposition, central-to-peripheral deposition ratio and clinical outcomes [28, 50].
Synopsis
There are a number of multi-dose DPIs that differ in design, mode of operation, and the steps required to perform a proper inhalation process. DPIs also differ in the manner and extent of feedback the user receives about the inhalation process. Only three DPIs – Novolizer®, Genuair® and NEXThaler® – provide feedback to the patient in the form of an auditory and visual signal confirming the correctness of the inhalation performed. Two DPIs – NEXThaler® and Spiromax® – have safeguards against dose accumulation in the inhalation chamber, while only the former one prevents dose loss due to patient non-administration. Feedback systems in multi-dose DPIs are important for the correct use of the inhaler, and thus for achieving the expected therapeutic effects. Efforts should be made to introduce DPIs to help patients assess the inhalation process, and thus eliminate errors.