Purpose
Endometrial cancer (EC) is the sixth most common type of cancer in women [1]. On the basis of histopathological, molecular, genetic, and biological parameters, it represents a very heterogeneous group of malignancies that in over 70% of cases are diagnosed at an early stage and older age [1, 2]. Total hysterectomy with bilateral salpingo-oophorectomy is the primary treatment modality for endometrial cancer in FIGO IA stage cases. Indications for adjuvant treatment are determined on the basis of pathohistological and molecular characteristics as well as age and general condition of the patient [3]. The most important factors for the progression of the disease in patients belonging to low- and medium-risk groups are the presence of lymphovascular invasion, depth of myometrial invasion, tumors larger than 2 cm, involvement of distal segment of the uterus, and clear cell or serous carcinoma [2, 4, 5].
Genetic analysis of endometrial carcinomas has helped stratify the risk of disease progression and further highlighted great heterogeneity of the tumors [6]. Therefore, it was found that the presence of POLEmut indicates a good prognosis regardless of clinical-pathological characteristics of the tumor [7]. In low-risk endometrial cancer patients, the presence of β-catenin (CTNNB) exon 3 hot-spot mutation indicates an increased aggressiveness of the tumor [8]. Klat et al. retrospectively examined the presence of L1 cell adhesion molecule (L1CAM) positivity in 312 tumor samples from patients suffering from FIGO stage I and II endometrial cancer. Results of this study showed an increased aggressiveness of L1CAM-positive tumors, but no effect on overall survival (OS) [9]. Although patients in the initial stage of the disease without the presence of risk factors for disease progression have a good prognosis, Stasenko et al. found the recurrence rate of 2.9% in the group of patients with ultra-low risk in low-grade tumors of endometrioid histology without invasion of the myometrium [8]. At the same time, in this research, no clinical or genetic characteristics were found to indicate an increased risk of relapse of the disease with statistical significance [9]. In recent years, due to the need to precisely define the indications for adjuvant treatment in the early stage EC, there has been a division made according to risk groups based on clinical, pathological, molecular, and genetic prognostic factors, which identify patients at risk for disease relapse, who may benefit from adjuvant therapy with an acceptable level of toxicity [10].
The aim of this research was to examine the clinical and morphological factors that influence disease-free survival (DFS) and five-year OS in FIGO IA stage endometrial cancer patients.
Material and methods
A retrospective clinical study was conducted, primarily based on the identification of factors that influence five-year DFS and OS in FIGO IA stage endometrial cancer patients. The research was conducted at the Center for Radiation Oncology of the University Clinical Center Kragujevac, after obtaining consent from the institution’s ethics committee. The study integrated data of FIGO IA stage endometrial cancer patients (2009 FIGO classification) [11], who presented to the gynecological oncology board for the first time after surgery treatment, from January 1, 2016 to January 1, 2022. Data were obtained by reviewing medical histories and consultative decisions. Criteria for inclusion into the study were as follows: histopathologically verified FIGO IA stage endometrial cancer based on decision of the gynecological oncology board, total hysterectomy with bilateral salpingo-oophorectomy, complete medical documentation, and existence of a certificate of survival. Exclusion criteria were incomplete medical documentation, inadequate initial definition of the stage of disease, not attending control examinations (3-6 months in the first two years, 6 months from 2-5 of the fifth year, then annually), histopathologically verified uterine sarcoma, and previously diagnosed tumor in the abdomen and/or pelvis. According to a decision of the gynecological oncology board, adjuvant brachytherapy was applied to patients with an initially higher risk of disease progression based on 2016 ESMO-ESGO-ESTRO classification [12], given that no molecular-genetic testing was available during the study. High-dose-rate endocavitary brachytherapy was applied according to a regime of 4 × 600 cGy or 3 × 700 cGy, once a week. A cylinder diameter from 20 to 30 mm was used, and the dose was prescribed at a depth of 5 mm from the vaginal surface, while reproducibility was checked during each application based on X-ray examination to plan the treatment. The dose was also calculated according to International Commission on Radiation Units and Measurements 38 (ICRU 38) for the bladder and rectum as well as the maximum dose in the vaginal mucosa [13]. Quality assurance procedures were assessed daily, weekly, and monthly in accordance with American Association of Physicists in Medicine (AAPM) task group No. 40 report [14]. The groups of patients treated with brachytherapy were additionally compared with those treated without brachytherapy.
Study variables
Independent variables
Overall survival of patients (starting from the date of surgery until the date of last control or death), occurrence of disease progression (time elapsed from surgery to the date of disease progression based on diagnostic imaging or pathohistological confirmation).
Dependent variable
Data from medical records, including pathohistological type, tumor histological and nuclear grade, presence of lymphovascular and myometrial invasion, involvement of uterine distal segment, comorbidities according to Charlson’s comorbidity, age-combined risk score [15], number of deliveries and abortions, brachytherapy dose, number of fractions, existence of adequate pre-operative magnetic resonance imaging (MRI) or computed tomography (CT) of the abdomen and pelvis, selective (in the sample less than 10 lymph nodes) or complete pelvic lymphadenectomy, type of institution where the surgery treatment was performed (secondary or tertiary), existence of disease progression (local, loco-regional recurrence, or distant metastases), and localization of recurrence or distant metastases, were obtained and assessed.
Anamnesis data related to patient’s habits, such as cigarette smoking and alcohol consumption as well as socio-demographic characteristics of patients, including age at the time of presentation to gynecological oncology board and place of residence (village or city) were also assessed. Inspection of medical documentation yielded data on socio-demographic characteristics of patients, associated diseases and previous treatment, pathohistological characteristics of the tumor, and proposed and implemented adjuvant therapeutic protocols. Incomplete data from patients’ medical histories were not analyzed. The length of survival at the moment of research was estimated after reviewing medical documentation (if the patient had visited a doctor in the previous six months), or by contacting patient or family member over the telephone.
The collected data were processed with descriptive statistics methods using measures of central tendency and standard deviation for continuous variables, with normal distribution and relative frequency for categorical variables. For continuous variables, the significance of differences was tested using parametric Student’s t-test and non-parametric Mann-Whitney U test in case of irregular data distribution. χ2 test was applied for categorical variables. The length of survival was analyzed with Kaplan-Meier method, while log-rank test was used to assess differences between groups. The difference in the compared data was considered statistically significant if probability of null hypothesis was less than 5% (r < 0.05). SPSS v.18 statistical software for Windows (Chicago, IL, USA) was applied for data calculating and processing.
Results
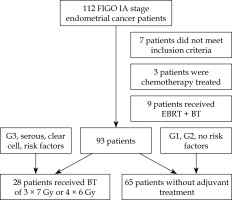
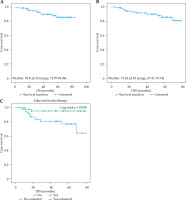
The study included 112 patients with pathohistologically verified FIGO IA stage endometrial cancer, initially treated with total hysterectomy with bilateral salpingo-oophorectomy. Seven patients, who did not meet the inclusion criteria were excluded as well as patients who received chemotherapy (3 patients) and those who underwent external beam radiotherapy (EBRT) + brachytherapy (9 patients). In total, data of 93 patients were included in the analysis, of which 28 were treated with brachytherapy (Figure 1). The results show that the overall 5-year OS was 89.2%, with a median survival of 76.8 ±2.13 months (range, 72.97-81.04 months) (Figure 2). The 5-year DFS was 88.2%, with a median of 72.62 ±2.10 months (range, 67.51-75.74 months) (Figure 2).
Fig. 2
Five-year overall survival (A), disease-free survival (B), and impact of brachytherapy use on DFS (C)
Socio-demographic characteristics of the patients showed the average age of 61.46 ±8.50 years, with 66.7% living in cities. In terms of habits, 37.6% of the patients smoked cigarettes, while 11.8% occasionally enjoyed the consumption of alcoholic beverages. The analysis showed that socio-demographic factors and habits were not associated with disease progression. Data from the patient’s reproductive history related to the number of deliveries indicated a statistically significant impact on DFS (Figure 3), while the number of abortions and presence of menopause at the time of diagnosis showed no impact on the length of DFS.
Fig. 3
Pre-operative staging (A), type on institution where the surgery was performed (B), number on deliveries (C), family anamnesis (D) Pathohistological type (E), lymphovascular invasion (F), impact on DFS

The results of this research show that intra-cavitary brachytherapy was performed in a third of all patients (30.8%) (Table 1). Progression of the disease was recorded in 11 patients according to RECIST 1.1. criteria, predominantly loco-regionally, while in one patient, the progression occurred in the liver [16]. Three patients experienced gastrointestinal manifestations due to acute radiation toxicity. The manifested toxicity was mild (grade 1), according to Common Terminology Criteria for Adverse Events (CTCAE) v. 5 (Table 1) [17].
Table 1
Analysis of the influence of clinical and morphological factors on disease-free survival time in FIGO IA stage endometrial cancer patients
Adjuvant treatment with 3-4 intra-cavitary brachytherapy applications with a dose per application of 600 to 700 cGy had a significant impact on extending DFS (Figure 1, Table 1). Considering the demonstrated impact of adjuvant brachytherapy, an additional analysis of patients treated with brachytherapy compared with patients treated without brachytherapy was performed (Table 2).
Table 2
Analyzes of the impact of brachytherapy in FIGO IA stage endometrial cancer patients
Pre-operative MRI diagnostics were done in 63.4% of the patients, and statistical analysis indicated a strong influence on DFS (Figure 2). Moreover, 59.1% of patients had their surgeries performed in secondary type of healthcare centers, while 40.9% of treatments were performed in tertiary type of institutions (Table 1). Pelvic lymphadenectomy was performed in 12.9% of patients, whereas selective lymphadenectomy was done in 8.6% of cases, but these treatments showed no statistical significance in DFS (Table 1).
Endometrioid adenocarcinoma was pathohistologically recorded in 90.3% of our study subjects, serous type in 3.2%, and clear cell form in 6.5% of the patients. Forms with high nuclear and histological grades made up the smallest share of tumors, i.e., 6.5% and 5.4%, respectively. The presence of lymphovascular invasion was verified in 22.6%, while myometrial invasion was more frequent and recorded in 64.5% of the patients. The involvement of distal part of the uterus was found in 15.1% of the patients. Pathohistological characteristics of the tumor, such as the histological and nuclear grade of the tumor were identified as statistically significant factors affecting the length of DFS (Tables 1, 2).
Discussion
The conducted study was based on the analysis of the length of five-year OS and DFS in FIGO IA stage endometrial cancer patients, who had previously undergone operative treatment. Endometrial cancer in the early stage is a disease with a relatively good prognosis, if the optimal therapeutic modality is applied according to risk factors for progression of the disease. According to data from the relevant available literature, the five-year OS is ranging from 80.0% to 91.6%, while the five-year DFS in patients with FIGO IA stage disease is over 85% [18-20]. Similar to the results of studies conducted in other centers, the five-year OS in our study was 89.5%, while during the same period, 88.2% of the patients were free of disease progression. Given that the frequency of disease recurrence in early stage ranges from 2.9% to as high as 28.6% [8, 21, 22], it was of particular interest to identify factors associated with shorter DFS.
Comorbidities and events from patient’s reproductive medical history are associated with the occurrence of endometrial cancer, but they can also have a certain influence on local control [23]. When these factors were considered, a statistically significant association was found between a higher number of deliveries and shorter DFS. A study by Lesko and colleagues conducted in the 1990s indicates that the older age of women at the time of their last childbirth has a protective effect on the occurrence of endometrial cancer [24]. Contrary to our results, a very large Swedish study that integrated about 2,600,000 women showed a protective effect of multiparity and older age in the last pregnancy [23]. However, when it comes to high-risk pregnancies with existing gestational hypertension associated with numerous inflammatory and immunological disorders, the contribution to the formation of this tumor cannot be ruled out with certainty [25]. Given that at the time of conducting the study, researchers did not have access to precise data from the reproductive history of all patients. Therefore, it is necessary to examine in detail which factors that cause multiparity are associated with DFS. In terms of genetic factors from family history, the most common were malignant and cardio-vascular diseases. The obtained findings imply that numerous genetic factors may be significant in long-term prognosis of the disease, which is not only related to personal characteristics of patients, but also to hereditary risk of certain diseases.
The modern approach to the treatment of gynecological malignancies foresees the implementation of precise indications for the application of adjuvant therapy, considering the expected long survival of patients in the initial FIGO IA stage of endometrial cancer and over-treatment risk. Previously conducted clinical studies suggest that adjuvant vaginal brachytherapy in early stages of endometrial cancer in a low-risk group does not significantly affect long-term control of the disease [26]. There are studies, in which adjuvant radiotherapy in FIGO stage I endometrial cancer patients was found to improve loco-regional disease control, but without affecting OS [18, 20, 27] (Table 3). According to data of Jeans et al., brachytherapy is an adequate form of treatment for patients with negative peritoneal cytology and clear cell, serous, or mixed carcinoma of the endometrium at an early stage [28]. Similarly, Shinde et al. observed that adjuvant brachytherapy in FIGO IA endometrial cancer patients with unfavorable histology improves OS [29]. The results of a recent retrospective non-randomized interventional study, which included 419 patients with FIGO stage IA, IB, and II endometrial cancer, suggest that patients with FIGO stage IA disease have the greatest benefit from the adjuvant treatment [22]. A study by Garzon et al. that integrated 4,156 FIGO IA endometrial cancer patients showed the non-exceptional effectiveness of vaginal brachytherapy in the medium-high risk group with less than two risk factors (grade 3, presence of lymphovascular invasion or non-endometrioid histology, without invasion of the myometrium) [26]. Additionally, results of a research by Jin et al. that included 238 high- and intermediate-risk patients (according to the ESMO-ESGO-ESTRO criteria) with FIGO stage I and II endometrial cancer, showed that the application of brachytherapy, as opposed to brachytherapy with EBRT, is a safe option in terms of disease control and manifestations of radiation toxicity [30]. Similarly, the current study showed that 3-4 applications of adjuvant brachytherapy with a therapeutic dose of 600 to 700 cGy has a statistically significant effect on DFS compared with the group without adjuvant treatment. According to data obtained from GOG-99 and PORTEC-1 study, the most common localization of disease relapse is the vaginal stump, with a frequency of 71.4% and 72.4%, respectively [27, 31, 32]. Likewise, in the present study, the highest frequency of vaginal recurrences and progression to regional lymph nodes was recorded, and one patient had distant liver metastases.
Table 3
Review of studies with similar design
| Authors (year) [Ref.] | Study sample | Brachytherapy regimen | Study outcome |
|---|---|---|---|
| Lin YJ et al. (2019) [20] | 84 FIGO I stage EC patients | 21 patients: EBRT (VMAT, 3D-CRT) 50.4 Gy/28 fx.; 46 patients: HDR-BT 2-8 Gy/2-6 fx.; 13 patients EBRT (50.4 Gy) + BT (2-8 Gy/2-6 fx.) | OS: non-RT group, 96.3% OS: RT group, 91.6% 5-year loco-regional recurrence rate: 2.0% Recurrence-free survival rate: non-RT group, 97.9% Recurrence-free survival rate: RT group 97.1% |
| Cisek P et al. (2018) [21] | 178 FIGO I stage EC patients | HDR-BT: 30 Gy (15-32)/3-4 fx. | Treated group: 5-year OS, 93.00% Observed group: 5-year OS, 95.12% Treated group: 5-year DFS, 95.58% Observed group: 5-year DFS, 93.98% Treated group: 10-year DFS, 95.58% Observed group: 10-year DFS, 89.7% |
| Michalak M et al. (2020) [22] | 419 FIGO IA, IB, II stages EC patients | BT alone (for FIGO IA patients); total dose-rate, 28 Gy (range, 0.0-37.5) | 108 patients: 32 (28.6%) FIGO IA endometrial cancer patients experience recurrence in 5-year follow-up BT in FIGO IA patients significantly (8.3 times) reduced the risk of recurrence |
| Garzon S et al. (2022) [26] | Total, 4,156 EC patients: 447 (10.8%) stage I endometrioid – early-stage high- intermediate | HDR-BT: total dose 30 Gy (15-32)/ 3-4 fx. | High-intermediate risk and high-risk endometrial cancer represent < 1% of all ECs Observation or brachytherapy may be adequate in stage I endometrioid ECs with < 2 factors among grade 3, LVSI without grade 3, or lymphovascular space invasion |
| Sorbe B et al. (2009) [27] | 645 low-risk FIGO stage IA-IB EC patients | HDR or LDR-BT 3.0-8.0 Gy/3-6 fx. | Recurrence rate: 4.0% Impact of post-operative BT on even loco-regional recurrence rate in patients with low-risk endometrial carcinoma |
| Jeans EB et al. (2021) [28] | 182 serous and clear cell, FIGO stage IA or IB EC patients | HDR-BT: 21 Gy/ 3 fx. | Cumulative incidence of any recurrence at 5 and 10 years was 11.6% (range, 7.2-18.6%) and 12.7% (range, 8.0-20.1%), respectively 10-year OS in all patients: 73.3% (range, 64.6-82.9%) |
| Shinde A et al. (2018) [29] | 5,711 patients FIGO stage IA of clear cell, papillary serous, and carcinosarcoma patients | BT: no doses or number of fractions provided | 3-year OS: 87% in BT patients vs. 78% in non-BT patients |
Current recommendations for the treatment of endometrial cancer require adequate pre-treatment MRI diagnostic processing. Daix et al. examined the concordance of pre-operative ESMO-ESGO-ESTRO risk stratification and definitive pathohistological diagnosis. The results showed that the risk was initially underestimated in most patients [33]. A study conducted by Rei et al. indicated that pre-operative MRI with high sensitivity can assess the depth of myometrial invasion [34, 35]. Pre-operative MRI parameters of diffusion and perfusion with a high probability of involvement of regional lymph nodes and infiltration of the myometrium, in addition to assessing the stage of the disease, can also indicate micro-structural features of endometrial cancer [36]. Considering that lymphadenectomy increases the risk of post-operative complications, and that the frequency of positive lymph nodes is below 5% in the low-risk group, in situations where there is an optimal perioperative-risk assessment, it is possible to safely avoid this procedure [18]. Careful prediction of favorable prognostic factors includes the presence of grade I or II endometrioid tumors, less than 50% of myometrial invasion, and tumors smaller than 2 cm, with adequate pre-operative MR imaging [18]. Ignatov et al. confirmed that lymphadenectomy did not contribute to longer survival in 868 patients diagnosed with FIGO I and II endometrial cancer belonging to intermediate- and high-risk groups [37]. The results of the present study demonstrated that the omission of pre-operative MRI assessment of the stage of the disease had a statistically significant effect on DFS, which can potentially indicate an initially underestimated stage of the disease. McGowan et al. observed that there is no statistically significant difference in OS and treatment outcome depending on whether patients’ surgery with early endometrial cancer was performed in a cancer unit or a cancer center, considering that a minimally invasive approach is preferred in early stages [38]. Unlike the study by McGowan, our study showed that there is a significant difference in the length of DFS in relation to the type of healthcare provided by the institution where the surgery was performed. Therefore, it was found that patients operated in a tertiary center showed a significantly better DFS. These differences may be attributed to different levels of education and access to treatment in gynecologic oncology centers in developing countries. The success of treatment contributes to the education of the entire multidisciplinary team (gynecologist, radiologist, pathologist, radiation, and medical oncologist). It is possible that some smaller centers lack the conditions necessary for performance of highly specialized therapeutic modalities. Creating a strategy to improve the level of education of doctors in smaller centers as well as defining the minimum of patients with a certain pathology, could improve the quality of healthcare.
This study has certain limitations. The main shortcomings of the conducted research include its unicentric and retrospective character, and a small study sample of female patients.
Conclusions
The results of the current study showed that the application of adjuvant brachytherapy in patients with high-intermediate and high-risk contributes to prolongation of DFS. Moreover, the existence of a pre-operative MRI assessment of the stage of the disease as well as the type of healthcare provided by the health institution where the surgery was performed, significantly affect DFS. It is expected that the results of this study will confirm that the above-mentioned factors may suggest the existence of an additional risk for disease progression in FIGO IA stage endometrial cancer patients, who require adjuvant treatment.