Introduction
Basal cell carcinoma (BCC) is the most common skin cancer in the Caucasian population [1]. This tumour belongs to the group of non-melanoma skin cancers (NMSC) and accounts for about 80% of all cases in this group of cancers [2]. According to the GLOBOCAN report from 2018 [3], the new cases of NMSC accounted for 5.8% of all cancers globally (1 042 056 cases). BCC shows little, typically local malignancy only. These cancers are hardly ever metastatic (ranging between 0.0028 and 0.55 of all BCC cases) [4]. It has been proven, however, that these tumours tend to be aggressive, infiltrating deep structures [5]. The BCC surgical treatment brings good results, however, the relapses are frequent (according to data from Poland the rate is 46%) [6]. Based on the morphology of the lesion, the following BCC types can be distinguished: nodular, ulcerating, sclerodermal plane, superficial type and pigment contains [7]. Environmental factors and primarily UV radiation play a very important role in the formation of BCC [8]. For this reason, the largest percentage of BCC is located on the scalp and face skin [9]. Both age and gender are also relevant: BCC is most common among men over 60 years of age [10]. The presence of BCC is also associated with immunosuppression [11].
Human papillomaviruses (HPV) are a group of viruses from the Papillomaviridae family. About 150 types belong to this group, some of them contribute to benign lesions (skin warts, genital warts), and some may cause malignancies such as cervical cancer, penile cancer, head and neck cancer, or skin cancer [12–14]. The role of HPV in the formation of cutaneous squamous cell carcinomas is clear and associated with viral proteins E6 and E7 [15]. Oncoprotein E6 binds to cell’s protein p53, which leads to ubiquitination (signal for degradation) followed by degradation of p53 [16]. Degradation prevents the p53 induction of apoptosis or inhibition of proliferation of infected cells. Oncoprotein E7 binds to cell’s protein pRb and induces its phosphorylation, thereby leading to inactivation of this protein [17]. Inactivation releases E2F, which then, migrates to the cell nucleus and induces transcription of proteins responsible for the cell’s entry into S phase.
It is also believed that infection caused by viruses from the genus Betapapillomavirus (β-HPV) might be, too, associated with the risk of BCC, but the spread of data on the prevalence of the virus in biopsies is large – it ranges between 8% and 70% [18–21].
Aim
The aim of the study was to assess the presence and diversity of β-HPV types in skin samples taken from the tumour and a fragment of the healthy skin from patients with basal cell carcinoma. Also, factors such as patient’s age, hair colour, skin type, type of tumour, its location and number of tumours were investigated to find the correlation with the presence of β-HPV infection.
Material and methods
Clinical specimens
The skin biopsies from all 73 patients (45 men and 28 women) with histopathologically confirmed BCC were included into this study. Material from each patient was collected either from the neoplastic lesions or healthy skin around the lesion (surgical margin). All biopsies were taken at the Dermatology Clinic of the University Hospital in Krakow. The biopsies were collected using sterile scalpels, and placed in 0.9% NaCl. Afterwards, all samples were stored at –80°C in individual Eppendorf tubes to perform HPV tests.
Additional data were collected to determine sex, age, hair colour and the tumour location.
The trial was approved by the Bioethics Committee of the Jagiellonian University.
β-HPV detection
DNA was isolated from each sample by a spin-column method (Genomic Mini Test, A&A Biotechnology, Poland). To confirm the isolation of human DNA and to check its quality, the β-actin gene was amplified using the method described by Szostek et al. [22].
To detect the infections caused by β-HPV, and genotyping of 25 types (HPV 5, 8, 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, 36, 37, 38, 47, 49, 75, 76, 80, 92, 93, 96), PCR with reverse hybridization assay (RHA) was performed. For this purpose, the RHA Kit Skin (beta) HPV (Diassay, Rijswijk, the Netherlands) was used.
A process of genotyping involved performing a one-step amplification reaction of the E1 β-HPV gene fragment using a set of PM primers containing a biotin tag. Then, biotinylated amplicons were hybridized with the oligonucleotide probes immobilized on membrane strips. The created hybrids were visualized by colour reaction. The HPV93 plasmid clone and water were used as positive and negative controls, respectively.
Statistical analysis
Statistical analysis was performed using the Statistica 13.3 software. The average age was calculated along with the standard deviation depending on the sex, BCC type, tumour dissemination, tumour location and presence of HPV infection (both in the tumour fragment, the healthy skin fragment and after adding the results from both fragments). For continuous data, the Shapiro-Wilk test was conducted to check the normality of distribution. The data did not indicate a normal distribution, so the U Mann-Whitney test was performed for their analysis. To find the correlation between qualitative data, the Pearson χ2 test was carried out. In order to clearly present the obtained data with a correlation of statistical significance < 0.05, they were additionally presented in the form of multi-division tables.
Results and discussion
Structure of the studied group
In 45.2% of all cases, the tumour developed in a place exposed to UV radiation (Table 1). Exposure to sunlight is one of the most commonly mentioned risk factors for BCC [1, 8]. According to the literature, 58.6% to 80% of BCC cases are related to UV exposure [1, 23, 24]. In the studied group the most common areas of BCC location were the face (45.9%) and torso (43.2%) (Table 1). A statistically significant correlation was also observed between the tumour site and its subtype (Tables 2, 3). Moreover, the study showed a statistically significant relationship between the UV exposure and BCC subtype (Tables 2, 3). The nodular subtype is the most common among UV-exposed lesions, and the superficial subtype is the most prevalent among non-UV-exposed lesions. These observations are in line with the literature data [25].
Table 1
Characteristics of the research group
Table 2
Interdependence of quality parameters in the research group
Table 3
Presentation of statistically significant quality parameters in the form of two-split tables
The highest percentage of examined patients had blonde (42.9%) and brown hair (39.3%). These data are in line with the literature because the light hair colour is associated with the light skin colour, which is a risk factor for developing skin cancers [26]. Hair colour is also significantly statistically correlated with the tumour site (Tables 2, 3). This may be related to the fact that almost all BCC cases involve people with Fitzpatrick’s I or II phototype [27].
β-HPV infection was detected in 40 out of 73 (55%) patients, 36 HPV infections were detected within the tumour, while 33 HPV infections were present in healthy skin surrounding the tumour (Table 1). For 27 patients, the infection was identified in both the healthy skin and tumour area. The infection in the area of healthy skin only was found in 7 patients whereas 8 patients had it in the lesion area. In the literature, the percentage of patients with BCC with a positive HPV test varies between 67.4% and 70% [19, 21].
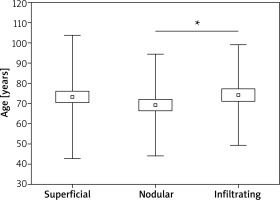
In this study, the average age of patients with BCC infected and uninfected by β-HPV does not differ statistically (Table 4). On this basis, it can be concluded that the age of the patients did not affect the presence of HPV infection. However, the correlation between the presence of HPV infection within the tumour and in the healthy skin is statistically significant (Tables 2, 3). Statistical analyzes showed that BCC subtypes differ in the study group by the average age of occurrence (Table 4, Figure 1). For nodular type it is 69.23±2.61 years, for superficial 72.65 ±15.91 years and for infiltrating 75.89 ±12.13 years. The difference is statistically significant between the nodular and infiltrating type (p < 0.05).
Table 4
Age difference of patients depending on selected parameters
BCC and HPV
Twenty three of the 25 β-HPV types tested were detected in the examined tumour samples. Both HPV15 and 47 types were not detected in any of the studied samples (Figure 2 A). The most frequent detected types were HPV24 (in 11 samples/patients), while HPV 9 and HPV 23 types were detected in 7 samples/patients, HPV 5 and HPV93 were detected in 6 patients. These results are consistent with the literature data. In the meta-analysis by Ramezani et al., HPV 24 is one of the most commonly detected type of HPV in BCC, while in the publication by Zakrzewska et al., this is the most commonly detected type [19, 28]. In the cited publications, other types mentioned were also often found in patients with BCC.
Figure 2
The number of patients: A – with specific types of virus detected, B – with specific types of virus detected separately in the tumour tine/location, separately in healthy skin C – with specific types of virus detected, depending on the type of tumour, D – depending on the type of tumour and the number of HPV types detected, E – depending on the number of tumours and the number of HPV types detected

Within the tumour, HPV 9, 23 and 24 was detected in 6 patients, HPV 5 and 17 in 4, while HPV 20 was the only one of the 23 types detected, which was not present in the tumour from any of the patients (Figure 2 B). In healthy skin surrounding the tumour, HPV 24 type was the most common (n = 6), whereas HPV 93 type was detected in 4 cases. No HPV 12, 14 and 49 types were found in patients’ healthy skin. These results are partly in line with the literature [19]. In the cited studies, HPV 24 was the most common tumour type, and HPV 5, 8, 9 and 93 were too, while HPV 5 was the most common in healthy skin, and HPV8, 24 and 93 types were also common [19].
The correlation between specific types of β-HPV viruses and subtypes of BCC was shown in Figure 2 C. For the superficial subtype (16/32 of HPV-positive cases), the most common type of HPV found was 24 (5 cases), types 9, 23 and 96 were also present (3 cases each). For the nodular subtype (15/22 of HPV-positive cases), the most common HPV types identified were 5 and 24 (5 cases each), and 9 and 23 types (3 cases each) were too. In contrast, for the infiltrating type (7/17 of HPV-positive cases), the most common HPV was 93 (4 cases). It was found that the percentage of HPV infection may differ between BCC types: in nodular it is higher than in superficial, but this result was not statistically significant [23].
Data regarding the number of samples uninfected with HPV, infected with one type of HPV or multiple types of HPV depending on the BCC subtype are presented in Figure 2 D.
In the case of the superficial type, mostly no HPV infection was found in 16 (50%) samples, an infection of one HPV type in 7 samples, and an infection with multiple types of HPV in 9 samples.
In the case of the nodular type, mostly an infection with multiple types of HPV was diagnosed (in 9 samples), no infection of any type of HPV in 7 samples, and an infection of one HPV type in 6 samples.
The infiltrating type had no infection of any type of HPV in 10 samples, an infection of one HPV type in 7 samples, and an infection with multiple types of HPV in no examined samples.
Data on the number of uninfected HPV cases, one-HPV type detected cases, and those with multiple HPV types, depending on whether the patient was diagnosed with a single or multiple tumour are also presented in Figure 2 E. The literature describes different types of HPV detected in BCC biopsies, and, also that the presence of mixed infections is much more common in BCC than in the healthy skin samples [23, 29, 30].
No statistical differences were found, which indicates that the infection with multiple types of HPV does not contribute to more common occurrence of skin cancer. Unfortunately, there are yet no data regarding this subject in the literature.
Conclusions
The study confirms the fact previously noted in the literature that in individuals diagnosed with BCC, HPV infections are present not only within the tumour but also in the healthy skin. The authors also noticed that infections with individual specific HPV types occur more often in specific BCC subtypes. In addition, preliminary studies suggest that one of the risk factors for development of infiltrating lesions is the presence of a single HPV 93 infection, but further research is required to confirm these assumptions.