Introduction
Actinic keratosis (AK) is a common skin disease that occurs in response to excessive exposure to ultraviolet (UV) radiation. This disease affects up to 60% of general population over 40 years of age, that is highly exposed to ultraviolet radiation [1]. Prevalence is dependent on many factors such as age, gender, latitude, fair skin (Fitzpatrick skin types I–III), but also immunodeficiencies or human papillomavirus infections [2].
Actinic keratosis is a disease characterized by the presence of hyperkeratotic, scaly papules or plaques, limited to the epidermis. Changes occur more often in places exposed to the ultraviolet radiation than other, e.g. the face, scalp and forearms. Actinic keratosis is considered to be a precancerous lesion that, in some cases, might lead to squamous cell carcinoma (SCC) [3]. Although the probability of a tumor transformation of singular lesions is quite low, it should be noted that patients who were highly exposed to the ultraviolet radiation have many AK changes, which is why the overall risk of squamous cell carcinoma is cumulative. It is estimated that a patient with 7–8 lesions of AK has a 6.1–10.2% risk of developing SCC within 10 years. In addition, 60–82% of SCCs are produced directly at or near the AK changes [4].
Unfortunately, it is not possible to assess which AK plaques will transform into SCC, thus it is important to treat AK lesions effectively.
Treatment regimens in AK could be divided into lesion-directed and field-directed therapies. Selecting a therapy for a particular patient must always be supported by a history of disease, the amount and localization of lesions, the overall level of sun damage, and the patient’s ability to cooperate and willingness to follow medical advice. Lesion-directed therapy consists of cryotherapy, curettage with electrostimulation and chemical peels. Cryosurgery with liquid nitrogen is one of the most commonly used methods in the US [5] and is dedicated to people with many, not very large and well-demarcated changes. Unfortunately, the common side effect of this therapy is hypopigmentation of the skin as well as pain during the treatment. In addition, changes tend to recur [6]. The field-directed therapy is targeted at people with multiple lesions located in similar localizations and with a subclinical AK. This approach includes methods such as dermabrasion, photodynamic and laser therapy, as well as topical agents: 5-fluorouracil, imiquimod, 3% diclofenac gel. A large limitation of topical application is their recommended time of use. Imiquimod, depending on the formulation, must be used for weeks/months, fluorouracil for weeks, and diclofenac for months [7]. In addition, according to the literature, 5-fluorouracil and imiquimod cause skin irritation that leads to a serious discomfort, intolerable to a number of patients. It results in discontinuation of the treatment. On the other hand, diclofenac can cause allergic reactions [8].
The best results in treating AK lesions have been achieved by combining lesion-directed and field-directed techniques. It is reported that combining diclofenac [9] with imiquimod [10] or 5-fluorouracil [11], before cryotherapy, is successful.
The new therapeutic option for the treatment of actinic keratosis is ingenol mebutate gel (0.015%, 0.05%). A 0.015% gel is intended for daily use on the face or scalp for 3 consecutive days, while a 0.05% gel is dedicated to patients with AK lesions in the extremities and trunk and should be applied once a day for 2 consecutive days. In our article we would like to present results of the treatment of 10 patients in the Department of Dermatology and Venereology of the Medical University of Lodz.
Aim
Retrospective evaluation of response and potential side effects of ingenol mebutate treatment in the clinical practice among Polish patients.
Material and methods
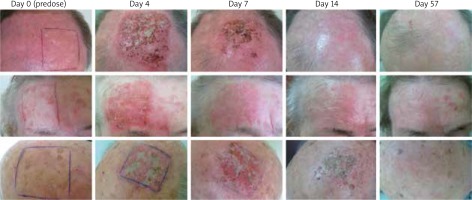
ten patients of the Department of Dermatology and Venereology of the Medical University of Lodz were qualified for ingenol mebutate treatment. All patients presented Fitzpatrick phototype I, II or III and had changes of actinic keratosis within the scalp (scalp or forehead). Patients were aged between 68 and 84 years. The study involved 9 men and 1 woman. Each patient presented an area of 25 cm2 occupied by AK changes. Patients with hypertrophic lesions, who recently used immunosuppressive or immunomodulatory medications, were excluded. Patients had been self-applying a 0.015% gel on the marked area for 3 consecutive days. Each of them was assessed at baseline and on day 4, 7, 14 and 57.
The effects of treatment are shown in Figure 1.
On day 57, all patients presented a complete absence of AK lesions in the area of ingenol mebutate application. In every treated patient there were observable local skin reactions that could be classified as erythema, flaking, crusting and swelling, of various intensity. The presence and intensification of those local reactions peaked on day 4. Two patients presented a severe local skin reaction with a bacterial superinfection. They received antibiotics: doxycycline (200 mg in two doses for 10 days) and fusidic acid (applied on the skin twice a day for 10 days). Four patients had moderate and other 4 mild local skin reactions. However, those symptoms were temporary and not troublesome for the patients.
Discussion
Ingenol mebutate is a diterpene ester, which is extracted from the plant called Euphorbia peplus. In the past, the sap of the plant was used as a remedy for many skin diseases [12].
Ingenol mebutate has multiple mechanisms of action. One of them is an initial chemoablation by disruption of the plasma membrane and mitochondria, which leads to a loss of mitochondrial membrane potential and, ultimately, necrosis of locally affected cells. The second mechanism of action is elimination of tumor cells by the tumor-specific antibodies, which proinflammatory cytokines and massive releasing of neutrophils – leading to neutrophil-and antibody dependent cellular cytotoxicity [13, 14].
Lebwohl et al. [7] in two of their studies confirmed a high efficacy of ingenol mebutate. Studies (n = 547) examined the ingenol mebutate 0.015% gel versus a vehicle for 3 days in AK on the face or scalp while other studies (n = 458) tested ingenol mebutate 0.05% versus a vehicle for 2 days in AK on the trunk or extremities. Patients had been applying the substance on AK areas of 25 cm2. The median reduction in the lesion count was 83% with 0.015% gel and 75% with 0.05% gel, compared to a 0% reduction in both groups using the vehicle. Among patients using ingenol mebutate on the scalp, the most common skin reaction was pain (13.9%), pruritus (8%) and irritation (1.8%). No serious adverse events were observed [7]. Moreover, Jim On et al. [15] analyzed a relation between AK lesions count at week 8 adjusted for baseline and composite LSR score (local skin reaction score). The percentage reduction in AK lesions was higher in patients with a higher LSR score. A large skin reaction after ingenol mebutate treatment gives more reliable probability of AK clearance [15].
The same clearance rate as after using ingenol mebutate for 2–3 days is achieved with other field therapies but it requires a longer treatment period. Using 5-fluorouracil in a 4-week treatment leads to a complete clearance rate of 43% [16]. A treatment with imiquimod causes complete clearance rates from 25% to 35.5% after 2 or 3 weeks’ treatment [17].
In another trial, patients received ingenol mebutate on approximately 250 cm2 sun-damaged skin for three consecutive days. Of 61 patients, 10 had a subnanomolar level of ingenol mebutate in whole blood (0.235–0.462 nM). No serious adverse reactions were observed, most of them were mild to moderate in intensity [18].
Ingenol mebutate could also be a concurrent treatment used in order to increase the effectiveness of the therapy. Hashim et al. confirm that application of the 0.05% ingenol mebutate gel on the same day as a cryosurgery is more effective in reducing numerous hyperkeratotic actinic keratosis lesions (–4.3 vs. –2.8 in the control group) and non-hyperkeratotic lesions (–3.8 vs. –0.8 in the control group). No significant increase in the number of local skin reactions was observed [19].
Erlendsson et al. demonstrated in a trial with hairless mice that ingenol mebutate prevents progression of UV-induced photodamages. Sixty hairless mice were exposed to the ultraviolet radiation and received five single treatments at 4 weeks’ intervals. On day 140, a standardized UV-damage scale (0–12) was lower in mice with ingenol mebutate treatment compared to UVR alone (UVR 10.25 vs. UVR + ingenol mebutate 6.0; p = 0.002). A topical usage of clobetasol propionate to reduce local skin reactions was also assessed. Surprisingly, clobetasol propionate increased LSR (max LSR Tx 1-5: UVR + ingenol mebutate + Clobetasol propionate 3.6–5.5 vs. UVR + ingenol mebutate 2.6–4.3) but at the same time provided better prevention of the photodamage [20].
The treatment with the ingenol mebutate gel could also be beneficial from a social point of view. In Greece, ingenol mebutate 0.05% and 0.015% were the most cost-effective topical treatment options, compared to diclofenac and imiquimod (incremental cost-effectiveness ratio of € 30,000 and € 10,868 per quality adjusted life-year QALY, respectively) [21].
Conclusions
To sum up, in our study, the 0.015% ingenol mebutate gel applied to the face or scalp once a day on 3 consecutive days was effective in treating actinic keratosis.
The big advantage of using ingenol mebutate is that we can achieve a big efficacy after only 2 or 3 applications. Many patients have problems with regular use which can lower effectiveness reported beforehand in clinical trials. A short-time treatment (like 2–3 applications) increases the probability of the patient compliance. Another benefit of a short-time usage is that local reactions disappear relatively quickly. On the face or scalp the peak of the local skin response was observed on day 4 but already on day 15, almost all of it has been resolved.
Our study and all reviewed studies show that ingenol mebutate is highly efficacious and has a generally temporary and mild to moderate local skin response. Even in cases where severe skin reactions appear, the final cosmetic effect is very good and satisfying to our patients. These findings suggest that ingenol mebutate could be a first-line field treatment for actinic keratosis.