Introduction
Skin inflammatory diseases, like contact dermatitis (CD) and psoriasis, affect millions of people worldwide, and subsequently affect the quality of their life [1]. The prevalence of CD is reported to range from 10% to 40%, in general [2]. The occupational CD is reported to represent 70–90% of all occupational skin diseases, what was described to deteriorate functional capacity and the quality of life of the patients [3].
Skin epidermis and the neural plate both originate from embryonic ectoderm, so the skin and central nervous system are embryologically related organs. It was described that stress may be considered as a precipitating factor in onset or exacerbation of skin diseases through psychosomatic mechanisms [4]. Some studies also reported that psychological stress might be triggering or worsening various skin diseases, like psoriasis, alopecia areata, vitiligo and atopic dermatitis [5, 6].
Stressful life situations were found to be related to the onset of symptoms in 16% of chronic urticaria cases [7]. The role of acute stress in CD was previously described [8]. Acute stress was described to enhance the inflammatory reaction of 2,4-dinitrofluorobenzene (DNFB)-induced contact hypersensitivity through the pro-inflammatory transcription factor NF-ƘB and IL-18 and the sympathetic nervous system mediated this response [8].
The effect of chronic stress with the subsequent depressive-like behaviour on CD was not previously addressed in experimental models. 2,4-dinitrofluorobenzene (DNFB)-induced contact hypersensitivity in mice is considered a well-established experimental model of human CD [9]. A recent meta-analysis demonstrated that the procedure of chronic unpredictable mild stress (CUMS) represents a robust animal model for depression that could significantly contribute to understanding of the underlying mechanisms of depression and the development of novel antidepressant drugs [10]. Therefore, these two models of depression and contact dermatitis were adopted in this study.
Contact dermatitis was described to be linked to allergy and was proved to be associated with inflammatory factors [11]. Therefore, treatments with the anti-inflammatory effect are potentially effective for treating CD. Pumpkin was described to have many health benefits like antioxidative, anti-inflammatory [12] and anti-fatigue activity [13]. The antidepressant-like effect of Sweetme Sweet Pumpkin™ (SSP) was previously reported as it increased the levels of brain-derived neurotropic factor (BDNF) [14]. In addition, some previous studies have proved the efficacy of pumpkin in healing of cutaneous diseases. Seeds oil extract of pumpkin (Cucurbita pepo L.) was proved to be effective in cutaneous wound healing in rats based on macroscopic, morphometric and histological investigations [15]. Peel extract of pumpkin (Cucurbita moschata Duchesne) was reported also as a natural remedy for treatment of burns that was attributed to antioxidative and antibacterial potential [16]. These studies were encouraging to test the efficacy of pumpkin in treating CD associated with depressive-like behaviour.
Aim
This study aimed to assess the efficacy of the extract of pumpkin fruit (Cucurbita pepo L.) in treating CD in an animal model of depression compared to a standard treatment of CD and explore the mechanism behind this effect.
Material and methods
Drugs
Betamethasone (BETA) was used, in this study, to treat the positive control group for pharmacological validation of the pumpkin extract (PE) cream. Betamethasone was used at a dose of 75 µg (thinly and gently painted) using a specific brush twice a day for 2 weeks.
Preparation of pumpkin extract (PE)
Fresh pumpkin (L. Cucurbita pepo) fruits were obtained from the Jeddah market in Saudi Arabia and were identified by a botanist at the Botany Department, Faculty of Science, King Abdulaziz University (KAU). Extraction of pumpkin was done according to Wang et al. [13]. The raw fruits together with the skin were cut with a slicer, after removing the seeds, and were dried by using a lyophilize machine freeze-drier (FD5508; ilShin Biobase Co., Ltd., Korea) and crushed by a grinding electrical machine. The powder was passed through a 40-mesh sieve in order to obtain the fine powder and to be stored in an airtight container.
The dried powder (50 g) was mixed with 450 ml of 80% ethanol for 1 day at 37°C temperature, left in a shaker machine (JSSI-100T; JS Research Inc., Compact Shaking Incubator., Korea) for 1 day and filtered with cotton and filter paper on the 2nd day. This extraction process was repeated twice and the excess solvent was removed using a rotary vacuum evaporator (HS-2005S; HAHNSHIN Scientific Co., Ltd., Korea) to give an ethanol extract. It was left at the fume hood for extra evaporation of ethanol, then the extract was dried in a freeze-drier machine (FD5508; ilShin Biobase Co., Ltd., Korea). PE was stored in a suitable container till use after being dissolved in distilled water at a dose of 100 mg/kg and administered by gavage once daily for 2 weeks [13].
Analysis of the pumpkin extract
The components of PE were identified by using Trace Gas Chromatography and Mass Spectrometer (GC–MS) (Thermo Scientific, Austin, TX, USA) with a direct capillary column TR–5MS (30 m 0.25 µm 9 0.25 µm film thickness).
Preparation of topical application of PE
A simple ethanolic extract of pumpkin was formulated in Vaseline (2% w/w) using a ceramic mortar and pestle and kept in a sterile container. The PE was stored in a suitable container till use as PE cream at a dose of 0.52 µl/mm2 according to Bardaa et al. [15].
Experimental groups and dosage
Forty male albino rats weighing 30–40 g were purchased from the animal house of the King Fahd Medical Research Center (KFMRC) and were utilized in this study. The rats were acclimatized in the laboratory conditions for 1 week, before starting the experiment. They were housed in plastic cages in an air-conditioned room at 22 ±1°C, offered the standard animal chow and water ad libitum. Ten rats were assigned as the negative control group (control) which left unexposed to neither stress nor CD. The other thirty rats were subjected to CUMS procedure as they were exposed to different types of stressors at different times during the day for 4 weeks in order to prevent habituation to the stressors. The CUMS procedure was fully described in previous works [17].
Induction of CD
A rectangular area (3 × 2 cm) on the dorsal surface of the rats was marked and hair over this area was shaved carefully with an electrical shaving machine. Contact dermatitis was induced in this demarcated area in all rats exposed to CUMS according to Simonetta et al. [18]. Briefly, rats were sensitized by painting 50 µl of (1-fluoro-2,4-dinitrofluorobenzene, DNFB) (0.1%, v/v) in acetone : olive oil 4 : 1 (AOO) onto the shaved dorsum of each animal for 3 consecutive days. Four days after sensitization, each rat was challenged by painting 30 µl of DNFB (0.2%, v/v) in AOO onto the dorsum every 2 days for 15 days. The painted areas were observed for signs of skin irritation for 2 weeks. These rats were then assigned into 3 groups (n = 10 each); the positive control (CD) group was treated with the vehicle (AOO), the CD + BETA group was topically treated with Betaderm cream, the CD + PE group was topically treated with PE cream. Topical treatments were performed using a special paint brush twice a day for 2 weeks.
Assessment of behavioural changes
In order to confirm the effect of CUMS, the forced swim test (FST) was conducted for all rats after 4 weeks, as was previously described by Yankelevitch-Yahav et al. and Ali et al. [19, 20]. The rat was left to swim in a glassy cylindrical container with 15 cm depth of water at 25 ±2°C. The rat was observed by a technician blind to the experiment groups for 6 min. The total time, in seconds, spent by the rat without mobility during the 6 min was determined. Immobility meant “the cessation of limb movement, except for the minor movement necessary to keep the rat afloat”.
Assessment of serum corticosterone, tumour necrosis factor-a (TNF-α), interleukin-6 (IL-6) levels
Blood samples were taken from the intra-orbital sinus after completing the 4 weeks of exposure to CUMS. Serum was obtained by centrifugation at 3 000 rpm for 15 min and was kept at –18°C.
Corticosterone (ALPCO Diagnostics, Orangeburg, NY, USA) and TNF-α, IL-6 (Quantikine R&D system, USA Kit) were assessed in the serum using enzyme linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions.
Assessment of TNF-α, IL-6, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in the skin
To assess the anti-inflammatory effect of treatment, the levels of cytokines TNF-α, IL-6, COX-2 and iNOS were measured in the skin. Samples of the affected skin were obtained and kept at –80°C for assessment of protein and gene expression. These frozen samples were homogenized, then centrifuged for 10 min at 5000 g and the supernatant was used for assessment of these cytokines using ELISA (Thermo Fisher, Vienna, Austria) to assess the levels of cytokines.
Assessment of malondialdehyde (MDA) in the skin and serum
The MDA level was assessed spectrophotometrically according to the method described by Gamal et al. [21] using the Thiobarbituric Acid Reactive Substances (TBARS) Assay Kit (Biodiagnostic; Egypt).
Assessment of superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT) in the skin and serum
To assess the antioxidant effect of topical PE, the levels of SOD, GPX and CAT were measured. SOD Assay Kit (Biodiagnostic; Egypt) enabled very convenient SOD assaying through reduction of nitro blue tetrazolium (NBT) to insoluble blue formazan according to the method described by Noda et al. [22].
GPX kit (Randox Labs, Crumlin, UK) was used to assess GPX level. To quantify CAT activity assay kits (Biodiagnostic; Egypt) were used as described by Noda et al. [22].
Quantitative real-time PCR (qRT-PCR)
Total RNA extraction has been done from the tissue samples using TriFast™ reagent (PeqLab, Germany, Cat No.: 30-2010) as described in the manufacturer’s protocol. The concentration of the purified RNA was estimated by NanoDrop 2000c Spectrophotometer (Thermo Scientific, USA). The extracted RNA from each sample was reverse transcribed using SensiFAST™ cDNA Synthesis Kit for RT-qPCR (Bioline USA Inc., USA, Cat No.: BIO-65053), following the manufacturer’s instructions. The synthesized cDNA was stored at –80°C until utilization for qRT-PCR.
The qRT-PCR reactions were performed using SensiFASTTM SYBR Lo-ROX Kit (Bioline USA Inc., USA, Cat No.: BIO-94002) on the Applied Biosystems 7500 real-time PCR detection system (Life Technology, USA). Gene specific primers for rat-GAPDH internal control, rat- TNF-α, rat- IL-6, rat-iNOS and rat- COX2 (designed by Primer3 software (v.0.4.0) [http://frodo.wi.mit.edu/] while their specificity were checked using NCBI/Primer-BLAST program. The primers were then purchased from Willowfort™ (UK) (Table 1). The PCR mixture was prepared as follows: 10 µl SensiFASTTM SYBR Lo-ROX Mix, 0.8 µl forward primer, 0.8 µl reverse primer, 2 µl template cDNA, and 6.4 µl nuclease-free water. The reactions mix were transferred to the thermal cycler that was previously programmed to an initial hold at 95°C for 2 min followed by 40 cycles of 95°C for 15 s, then 60°C for 30 s. A negative control reaction containing no template was run in each experiment.
Table 1
The rat gene specific primers used in qRT-PCR
Melting curve analysis was carried out to prove specificity of PCR products and the Ct value for each reaction was obtained from amplification plots. The relative quantification for each gene expression in the tissue samples was calculated using comparative threshold (ΔΔCt) method with the GAPDH as the internal control gene. For overall fold change, it was calculated and linearized by the 2–ΔΔCt arithmetic formula.
Histological techniques
After finishing all treatments, rats were decapitated under anaesthesia with diethyl ether. The chest and the heart were opened and blood samples were obtained from the heart. Skin samples (2 × 2 mm) were dissected out and fixed in 10% neutral buffered formalin, then processed into paraffin blocks. The latter were sectioned at 4-µm to be stained with Haematoxylin and Eosin (H&E) and Masson trichrome for identification of collagen fibres.
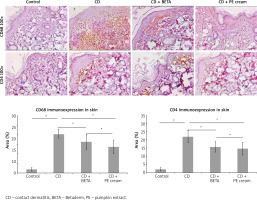
Immunohistochemical staining was performed using the streptavidin–biotin–peroxidase technique. Anti-CD68 antibodies (Biocare Medical, Pacheco, USA, at a 1/100 dilution), Anti CD4 (Biocare Medical, Pacheco, USA, at a 1/100 dilution) and anti-COX-2 (Biocare Medical, Pacheco, USA, at a 1/100 dilution) were used. The secondary antibody IgG was added while the primary one was omitted during staining of some slides in order to act as negative control slides. Counterstaining was performed using haematoxylin. Positive reaction was indicated by brown cytoplasmic staining. Photographing was performed using Olympus Microscope BX-51 supplied with digital camera. Pro Plus image analysis software (Media cybernetics, USA) was utilized to perform morphometric measurements as well as assessing antibody immunoexpression. Area percentage of immunoexpression of CD68- and CD4 and COX-2 was assessed in 30 fields using a 40× objective lens and 10× ocular lens and used as an indicator of the extension of the reaction. Epidermal thickness was measured in at least five fields from each slide and the mean was calculated for each animal.
The histopathological scores for the contact allergic dermatitis were performed using the scoring system previously described by Wang et al. [23]. This scoring system includes two parameters; infiltration of inflammatory cells (Grade 0, no changes; Grade 1, few infiltrations; Grade 2, moderate infiltrations; and Grade 3, extensive infiltrations) and cuticulate epidermis (Grade 0, no changes; Grade 1, minor keratinization in epidermal tissue; Grade 2, obvious epidermal keratinization; and Grade 3, severe keratinization in epidermal tissue).
Statistical analysis
Statistical Package of Social Science Program (SPSS, SPSS Inc., Chicago, Illinois, USA) version 20 was used to analyse the raw data. Results were presented as mean ± standard deviation (SD). The F test (one-way analysis of variance) was used to compare the studied groups followed by Post hoc test with the least significant difference to compare each two groups and avoid repeated comparisons. Significance was considered when p < 0.05.
Results
Analysis of the pumpkin extract
The constituents of PE were determined using GC-MS and were found to include mainly Oleic acid (about 56%), Hexadecanoic acid (about 9%), Decenoic acid (5%) and other components while the Linoleic acid represents only about 1% (Table 2).
Table 2
Components of L. Cucurbita pepo extract identified using gas chromatography and mass spectrometer (GC–MS) analysis
Effect of exposure to CUMS
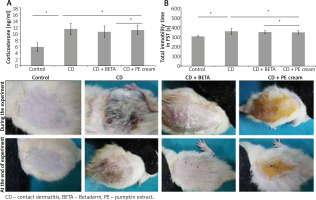
After 4 weeks of exposure to CUMS, the development of depressive-like behaviour was confirmed in the rats through assessment of behavioural changes and serum corticosterone level. A significant (p < 0.001) increase in the mean of immobility time was recorded in all CUMS-exposed rats compared to the controls. In addition, there was a significant (p < 0.001) increase in the serum corticosterone level in CUMS-exposed rats. Neither BETA nor PE cream affected significantly the total immobility time or the serum corticosterone level (Figure 1 A).
Effect on the morphologic appearance of contact dermatitis
Painting of the dorsal skin of rats with DNFB for 2 weeks resulted in signs of CD in the form of hardness, dryness, erythema and scaling. Application of BETA and PE to the affected areas for 2 weeks progressively improved these changes compared to the untreated group (Figure 1 B).
Effect on the inflammatory cytokines in the skin and serum
It was noticed that serum TNF-α, IL-6 levels were significantly increased (p < 0.001) in the untreated CD group compared to the controls. Both serum TNF-α, IL-6 showed no significant differences in either BETA- (p = 0.44, p = 0.08) or PE-treated (p = 0.26, p = 0.06) groups, respectively (Figure 2 A). The levels of IL-6, iNOS, COX-2, TNF-α in the skin significantly increased (p < 0.001) in the untreated CD group compared to the controls, while they were significantly reduced in BETA- (p < 0.001, p = 0.02, p = 0.03, p = 0.03) and PE-treated (p < 0.001, p = 0.03, p = 0.02, p < 0.001) groups, respectively, compared to the untreated CD group. There was no significant difference in levels of IL-6 (p = 0.49), iNOS (p = 0.97), COX-2 (p = 0.11), TNF-α (p = 0.71) in the skin of BETA- and PE-treated groups (Figure 2 B).
Figure 2
Pumpkin extract attenuates the proinflammatory cytokines secretion in DNFB-induced contact dermatitis. Levels of IL-6, TNF-α in the serum (A) and in the skin (B) were assessed using ELISA. The levels of mRNA of IL-6, iNOS, COX-2, TNF-α (C) were assessed using real-time PCR in the skin. Cox-2 immunoexpression (D, E) in the skin was assessed immunohistochemically
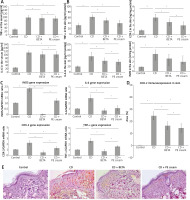
Gene expression of IL-6, iNOS, COX-2, TNF-α in the skin was assessed using qRT-PCT and their results were confirmatory to those in the ELISA. The mean expression of mRNA of IL-6, iNOS, COX-2, TNF-α in the skin was significantly up-regulated (p < 0.001) in the untreated CD group, while it was significantly down-regulated (p < 0.001) in BETA- and PE-treated groups compared to the untreated CD group with a significant difference (p < 0.001) between BETA- and PE-treated groups (Figure 2 C).
Immunoexpression of Cox-2 in the skin was also assessed immunohistochemically. It was noticed that Cox-2 expression was significantly up-regulated (p < 0.001) in the untreated CD group while it was significantly down-regulated (p < 0.001) in BETA- and PE-treated groups with no significant difference (p = 0.18) between the two groups (Figures 2 D, E).
Effect on MDA in the skin and serum
Contact dermatitis resulted in a significant increase in MDA in the skin as well as an insignificant increase in the serum. Although the DETA-treated group did show a significant change in the MDA level, the PE-treated group showed a significant decrease in its level in the skin (Table 3).
Table 3
Effect of L. Cucurbita pepo on the oxidant/antioxidant profile in the serum and skin
[i] One-way analysis of variance (ANOVA) was used to compare the studied groups followed by Post hoc test with the least significant difference. Results were presented in the form of mean±standard deviation (SD). Significance was considered when p < 0.05. P1 – significance versus the control, P2 – significance versus the CD, P3 – significance versus the CD + BETA.
Effect on SOD, GPX, CAT in the skin and serum
It was noticed that CD significantly reduced SOD (p < 0.001), GPX (p < 0.001) and CAT (p < 0.001, p = 0.002) in the skin and serum, respectively. Although the DETA-treated group did not show any significant change in SOD, GPX and CAT levels in either the skin or serum, the PE-treated group showed a significant increase (p = 0.04, p = 0.01, p = 0.02) in SOD, GPX and CAT in the skin but not in the serum, respectively (Table 3).
Effect on histological changes of the skin
Histopathological examination of the H&E stained sections revealed intact epidermis and dermis of the skin of the control group. On the other hand, the DNFB-induced CD groups showed a set of pathological changes including epidermal hyperplasia and vacuolation of keratinocytes as well as oedema in the superficial dermis, inflammatory cell infiltrate and increased capillaries and haemorrhages in some areas. Statistical analysis showed a significant increase (p < 0.001) in epidermal thickness as well as the histopathological score of CD in this group compared to the controls. A significant increase (p < 0.001) in the area percentage of Masson-stained dense collagen fibres in the dermis was also observed (Figure 3).
Figure 3
Pumpkin extract affects the DNFB-induced dermatitis in rats histologically. The histological changes in contact dermatitis (CD) group includes epidermal hyperplasia (EH), oedema in superficial dermis (star), vacuolation of keratinocytes (arrow), inflammatory cell infiltrate (arrow head) and increased capillaries and haemorrhages (bifid arrow) as well as increased dense collagen fibres (C) in the dermis are observed. The histological changes are improved in the treated groups (H&E and Masson stain)
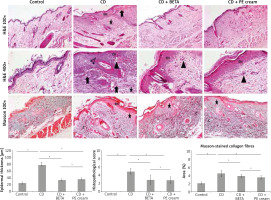
Pumpkin extract administered as a topical cream markedly improved CD-associated histological changes. BETA- (p < 0.001) and PE-treated (p < 0.001) groups showed a significant decrease in epidermal thickness as well as the histopathological score of CD compared to the untreated CD group with no significant difference (p = 0.25, p = 0.08) between the two groups, respectively. A significant decrease in the area percentage of Masson-stained dense collagen fibres in the dermis was recorded in BETA- (p = 0.01) and PE-treated (p < 0.001) groups with no significant difference (p = 0.16) between the two groups (Figure 3).
Inflammatory cell infiltrate observed in the superficial dermis was specified and quantified immunohistochemically and found to include mainly CD68-positive macrophages and CD4-positive T lymphocytes. It was noticed that DNFB-induced CD was associated with upregulation of CD68 and CD4 immunoexpression compared to the controls. On the other hand, CD68 and CD4 immunoexpression was significantly downregulated in BETA- (p = 0.002, p < 0.001) and PE-treated (p < 0.001) groups, respectively, compared to the untreated CD group with no significant difference (p = 0.07, p = 0.44) between BETA- and PE-treated groups, respectively (Figure 4).
Discussion
The interaction between the peripheral immune response and central nervous system can be evaluated in the skin; the accessible organ. Many studies proved that stress has a crucial role in many immunological diseases [24]. Stress influences the nature of some skin inflammatory diseases as psoriasis and atopic or allergic contact dermatitis [25]. This study was conducted to test the ability of the extract of pumpkin fruit to biochemically and histopathologically improve CD in chronically stressed rats and explore the mechanism behind this effect.
In this study, a depressive like-behaviour was induced in rats through exposure to CUMS and was confirmed by the significant prolongation in immobility time of FST as well as the significant increase in the serum corticosterone level. FST is described as an essential test in the study of neurobiological signalling pathways involved in depression [26]. It is sensitive to all available antidepressant drugs, therefore, commonly used to test the antidepressant effect of new drugs [27].
TNF-α is important for lipid and protein synthesis in the epidermis and consequently affects the composition and organization of skin barrier lipids, therefore, it is considered one of the inflammatory mediators implicated in inflammatory skin disease as atopic dermatitis [28]. Although TNF-α is present in the healthy skin, it was reported that activated macrophages, T cells, and keratinocytes synthesize and release it into circulation in cutaneous inflammatory conditions [29]. iNOS and COX-2 were also reported to have significant roles in the regulation of inflammatory reactions including those of the skin [30, 31]. Therefore, these proinflammatory cytokines were measured in this study to assess the anti-inflammatory effect of PE in the skin.
DNFB-painted dorsal skin appeared hard, dry and had scales indicating occurrence of contact dermatitis. This was associated with increased inflammatory cytokines TNF-α, IL-6 in the serum as well as IL-6, iNOS, COX-2, TNF-α in the skin. The results of the qRT-PCT confirmed increased mRNA expression of IL-6, iNOS, COX-2, TNF-α in the skin. These findings were supported by those previously reported in patients with acute and subacute allergic CD who had significantly increased TNF-α, IL6 serum levels compared to the healthy controls [32]. Inflammatory cytokines, such as IL-1b, IL-6, iNOS, COX-2, TNF-α and MMP-3, biomarkers that indicate inflammation severity, were significantly increased in the skin lesions and serum samples of the rat model of CD [33].
In this study, topical application of PE extract to the areas affected with CD resulted in marked improvement of hardness, dryness and scaling to a comparable degree to that treated with Betaderm cream, the standard treatment of CD. This morphological improvement was associated with a significant reduction in the inflammatory cytokines TNF-α, IL-6 in the serum as well as IL-6, iNOS, COX-2, TNF-α in the skin. These findings were in agreement with those of Kim et al. [14]. SSP significantly reduced the protein levels of TNF-α and IL-6 in the serum of depressed animals. These cytokines have been reported to be elevated in patients with depression and mice showing behavioural despair [34, 35]. Although the inflammatory response plays a chief role in the protection of the host and tissue repair, it can also damage the normal skin tissue [36], inhibition of pro-inflammatory cytokine expression was described to improve dermatitis as it protects from extended adaptive immunity [37].
DNFB-induced CD, in this study, was associated with a disturbed oxidants/antioxidants profile presented with increased MDA and decreased SOD, GPX and CAT in the skin and serum. In accordance with that Kaur et al. [32] recorded a significant upregulation in the oxidants pool and downregulation in antioxidative capacity during their study of patients with restricted allergic CD. This was attributed to the consumption of radical-scavenging antioxidants as a result of increased free radical amounts [38]. The PE-treated group showed a significant decrease in MDA level in the skin as well as a significant increase in SOD, GPX and CAT levels in the skin. The antioxidant properties of both pumpkin fruit and seed oil extracts were demonstrated previously [16, 39, 40].
DNFB-induced CD manifested histopathologically, in this study, with epidermal hyperplasia with subsequent increase in epidermal thickness, inflammatory cell infiltrate and dense collagen fibres. This was in agreement with those reported by Zhou et al. [41] in dinitrochlorobenzene-induced allergic CD in BALB/c mice, Kim et al. [42] in Benzene-induced skin irritation in rats [33] and in oxazolone-induced CD in mice. Significant up-regulation of Cox-2 immunoexpression in the CD group was among the findings in this study as well as previous studies [37].
It was previously documented that T lymphocytes and macrophages have crucial roles in skin inflammatory diseases such as CD and psoriasis [33]. Therefore, the immunoexpression of CD4-T lymphocytes and CD68-positive macrophages was measured in this study and was noticed to show a significant increase in DNFB-induced CD compared to the control. Skin keratinocytes act as a potent source of pro-inflammatory cytokines and chemokines which represent a dialogue between keratinocytes and activated immune cells and subsequently initiate and maintain the T cell-mediated immune responses in inflammatory lesions [43]. The T cells in the skin tissue were reported to sensitize and elicit a hypersensitive inflammation reaction [44]. These cellular events explained the keratinocytes hyperplasia and the associated overexpression of proinflammatory cytokines confirmed biochemically on the protein and gene levels as well as the up-regulation of immunoexpression of CD4-positive T lymphocytes and CD68-positive macrophages observed in CD group in this study.
Pumpkin extract administered as topical cream, in this study, markedly improved CD-associated histological changes to a comparable degree to that of BETA cream. CD68 and CD4 immunoexpression was significantly downregulated in BETA- and PE-treated groups which implied reduced hypersensitivity reaction [45]. No previous studies were found to describe the effect of pumpkin extract on CD associated with depression or chronic stress. On the other hand, some studies revealed an encouraging effect of pumpkin extract in wound healing like that one conducted by Bardaa et al. [46] and revealed the healing efficiency of the extracted oil of pumpkin (Cucurbita pepo L.) in deep second-degree burns in rats. Regarding the effect of pumpkin on treating CD, Zhang et al. [47] conducted a study on the effect of Caffeic acid (3,4-dihydroxycinnamic acid, CA), one of the six phenolic acids detected in pumpkin (Cucurbita maxima), on 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-induced CD. They found that CA has anti-inflammatory activities in both acute and chronic contact dermatitis models through blocking of mRNA and protein synthesis of the cytokines TNF-α, IL-6 and IL-1b and neutrophil-mediated myeloperoxidase activity [47].
Oleic acid (56%) represents the main constituent of pumpkin fruit extract, utilized in this study, while Linoleic acid represents only about 1%. These findings are in partial agreement with those of Bardaa et al. and Kim et al. [15, 48] who found that palmitic acid, oleic acid and linoleic acid represent the main fatty acids in pumpkin (Cucurbita pepo L.) oil seeds and account in total for about 90%. In their recent study, Bora et al. [49] reported that pumpkin (Cucurbita pepo) seed oil possesses a lipid profile, which comprises of 43–53% of linoleic acid and two classes of antioxidant compounds, namely; tocopherols and phenolics, which account for 59% of the antioxidant effects.
Improved CD, observed morphologically and evident both biochemically and histopathologically in the PE-treated group, might be attributed to antioxidant and anti-inflammatory effects of pumpkin that were also confirmed in this study. Altan et al. reported that the antioxidant effect of any drug prevents cell damage, promotes DNA synthesis, increases vascularity, increases the strength of collagen fibres and in fine improves the viability of collagen fibrils [50]. Thus, the antioxidant effect could be a mechanism contributing to treating CD.
Conclusions
Topical application of pumpkin fruit extract was effective in attenuating inflammation and oxidative changes associated with contact dermatitis and enhanced by exposure to chronic stress-induced depression. These results imply that pumpkin can alleviate symptoms of contact dermatitis as evident histopathologically through the down-regulation of pro-inflammatory cytokines and enhancing the antioxidant status in the skin. Therefore, pumpkin extract, applied topically in contact dermatitis, could be an alternative as well as preventive approach in treating CD. Further studies to explore the detailed mechanism of these effects and test them on volunteer patients of contact dermatitis are recommended.