Summary
There is no need for oral anticoagulation after the left atrial appendage occlusion procedure. Discontinuation or oral anticoagulation (OAC) continuation has a similar effect on stroke rate. Discontinuation or OAC continuation has a similar effect on bleeding rate. Discontinuation or OAC continuation has similar safety endpoints and efficacy.
Introduction
Percutaneous left atrial appendage occlusion (LAAO) has emerged as an acceptable alternative treatment for patients with nonvalvular atrial fibrillation (NVAF) in whom oral anticoagulation (OAC) therapy is contraindicated or anticoagulation therapy is ineffective [1–4]. Consequently, the number of LAAO procedures has rapidly increased in recent years.
As the left atrial appendage is the source of thrombus in 90% of patients with NVAF [5, 6] following LAAO, anticoagulation therapy may not be required. Currently, recommendations for postprocedure anticoagulation depend on the system used, varying from no treatment to life-long combination therapy with OAC and antiplatelet agents [1, 2, 7, 8]. Extended anticoagulation therapy should be considered when an endocardial system is employed (due to the thrombogenic nature of the occlusion device) [9], if the patient is at high risk of thromboembolic events or has experienced a stroke whilst on OAC. One further factor influencing the variability in prescribing practices may be that LAAO procedures are fairly new and may be relatively under-recognized by general internal medicine physicians.
Therefore, the optimal duration and the type of OAC treatment for patients with NVAF following LAAO remain unclear.
Aim
This study aims to address this problem by presenting the clinical outcomes of anticoagulation strategies in patients with NVAF after LAAO over more than 5.5 years of follow-up.
Material and methods
A prospective, single-center study was performed in 139 consecutive patients with NVAF who underwent LAAO with the LARIAT device (SentreHEART Inc, Redwood, CA) between December 2009 and December 2010. Inclusion and exclusion criteria for the LARIAT procedure have been described previously [10]. Patient data were collected for demographics, medical history, stroke risk (CHADS2 and CHA2DS2-VAS scores), bleeding (HAS-BLED score), and LAA dimensions. The study was approved by the local Ethics Committee and the Polish Ministry of Health.
Postprocedure anticoagulation
Aspirin monotherapy was recommended as postprocedure antithrombotic therapy. However, the final treatment was individualized based on the patients’ history, contraindications, risk of stroke and bleeding, and the preferences of both the patients and the treating physicians.
The cohort was divided into 2 groups. The No-OAC group patients received no anticoagulation or antiplatelet agents (aspirin or clopidogrel), whilst the OAC group received anticoagulation therapy with vitamin K antagonists (VKAs) (warfarin or acenocoumarol) or a new anticoagulant (NOAC) (dabigatran or rivaroxaban).
Thromboembolism reduction calculation
Similar to our previous study [11, 12], adverse events were reported during follow-up visits based on the Munich LAA consensus document [13]. We recorded cardiovascular, noncardiovascular, procedural and immediate procedural mortality, thromboembolic events (stroke, transient ischemic attack (TIA), systemic embolism), life-threatening, disabling or major bleeding events, including fatal bleeding; symptomatic bleeding in a critical organ, such as intracranial, intraspinal, intraocular, or intramuscular bleeding with compartment syndrome; bleeding causing hypovolemic shock or severe hypotension requiring vasopressors or surgery; overt source of bleeding with a drop in hemoglobin ≥ 5 g/dl or whole blood or packed red blood cell (RBC) transfusions of ≥ 4 units; and mortality. The efficacy of the procedure to prevent thromboembolic events (stroke, TIA, systemic embolism, or thrombus in the heart chamber) was calculated by comparing the actual event rate with the event rate predicted by the CHA2DS2-VASc scores [2, 3, 14]. The annual risk of individual patients was recorded, and the average annual risk for the entire study population was calculated. Thromboembolism reduction was calculated as follows: (estimated % – actual % event rate)/estimated % event rate) [15].
Bleeding reduction was assessed using the same method as that used for stroke reduction. The total number of major bleeding events per year was compared with the number of events predicted by the HAS-BLED score [16]: (estimated % – actual % event rate)/estimated % event rate) [15].
Statistical analysis
Continuous variables were analyzed for normal distribution using the Shapiro-Wilk test. Data are expressed as the mean ± standard deviation or median (interquartile range; Q1 – 25th percentile and Q3 – 75th percentile), unless otherwise stated. If a nonparametric test was used, the obtained data were additionally presented as the mean ± standard deviation for a better comparison with other studies. To assess the differences between two continuous variables, Student’s t-test (for normally distributed values) or the Mann-Whitney U-test (values not normally distributed) were used. Categorical variables were expressed as counts and percentages. Baseline characteristics between the groups were compared using the t-test for continuous variables and the χ2 test for categorical variables. Kaplan-Meier analysis was performed to estimate survival over time. Statistical analyses were performed with Statistica 10.0 (StatSoft, Tulsa, OK, USA). A two-sided p-value < 0.05 was considered statistically significant.
Results
The LAAO procedure was performed in 139 patients. One year after the procedure, 12 patients were lost to follow-up. Based on the data obtained from the Ministry of Health, it was possible to determine the status of the patients who were lost to follow-up. At the date of the last follow-up visit, 10 patients were still alive, and 2 patients had died.
At the end of the follow-up period, there were 52 (41%) patients in the No-OAC group and 75 (59%) patients in the OAC group. Median follow-up was 51 (43–57) months in the No-OAC group and 55 (48–59) months in the OAC group. The total follow-up duration for the study population was 532.7 patient-years. There were no differences in age, sex, comorbidities, LAA anatomy and dimensions, median follow-up, CHADS2 and CHA2DS2-VAS scores between the groups (Table I). The median HAS-BLED score was significantly lower in the No-OAC group than in the OAC group (3 (2–4) vs. 3 (3–4) (p = 0.014)) (Table I). There were no significant differences in the preprocedure anticoagulation regimens: in the No-OAC group, 81% of patients received VKAs, 13% antiplatelet agents and 6% no anticoagulation; in the OAC group, 87% of patients received VKAs and 13% antiplatelet agents. There were significant differences in the indications for LAAO between the No-OAC and OAC groups: LAAO was performed in 8% vs. 28% of patients with a history of a prior stroke/TIA while receiving OAC; in 23% vs. 10% of patients because of failure/complication while receiving OAC (other than stroke/TIA); 27% vs. 6% of patients because of contraindications to OAC; 42% vs. 51% of patients because of labile international normalized ratio (INR) (all p < 0.001), respectively.
Table I
Baseline clinical characteristics
| Parameter | No-OAC group (n = 52) | OAC group (n = 75) | P-value |
|---|---|---|---|
| Age [years] | 60.7 ±10.2 | 62 ±8.6 | 0.21 |
| Median follow-up [months] | 51 (43–57) | 55 (48–59) | 0.19 |
| Sex (female) | 23 (44%) | 35 (47%) | 0.92 |
| Median CHADS2 score: | 1 (1–2.5) | 2 (1–3) | 0.10 |
| CHADS2 score 0 | 1 (2%) | 2 (3%) | |
| CHADS2 score 1 | 29 (56%) | 28 (37%) | |
| CHADS2 score 2 | 9 (17%) | 25 (33%) | |
| CHADS2 score 3 | 12 (23%) | 14 (19%) | |
| CHADS2 score 4 | 1 (2%) | 6 (8%) | |
| CHADS2 score 5 | 0 | 0 | |
| CHADS2 score 6 | 0 | 0 | |
| Mean CHADS2 – score | 1.67 ±0.9 | 1.9 ±1.0 | – |
| Median CHA2DS2-VASc score: | 2 (1–4) | 3 (2–4) | 0.74 |
| CHA2DS2-VASc score 1 | 15 (29%) | 14 (19%) | |
| CHA2DS2-VASc score 2 | 15 (29%) | 20 (27%) | |
| CHA2DS2-VASc score 3 | 7 (13%) | 12 (16%) | |
| CHA2DS2-VASc score 4 | 6 (12%) | 12 (16%) | |
| CHA2DS2-VASc score 5 | 6 (12%) | 13 (17%) | |
| CHA2DS2-VASc score 6 | 3 (6%) | 3 (4%) | |
| CHA2DS2-VASc score 7 | 0 | 1 (1%) | |
| CHA2DS2-VASc score 8 | 0 | 1 (1%) | |
| CHA2DS2-VASc score 9 | 0 | 0 | |
| Mean CHA2DS2-VASc score | 2.6 ±1.6 | 3.1 ±1.7 | – |
| Median HAS-BLED score: | 3 (2–4) | 3 (3–4) | 0.014 |
| HAS-BLED score 1 | 8 (15%) | 2 (3%) | |
| HAS-BLED score 2 | 12 (23%) | 12 (16%) | |
| HAS-BLED score 3 | 17 (33%) | 32 (43%) | |
| HAS-BLED score 4 | 9 (17%) | 20 (27%) | |
| HAS-BLED score 5 | 3 (6%) | 6 (8%) | |
| HAS-BLED score 6 | 3 (6%) | 3 (4%) | |
| Mean HAS-BLED score | 2.9 ±1.3 | 3.3 ±1.0 | - |
| Previous CVA/stroke | 12 (23%) | 19 (25%) | 0.90 |
| Hypertension | 47 (90%) | 72 (96%) | 0.36 |
| Coronary artery disease | 8 (15%) | 15 (20%) | 0.42 |
| DM 2 | 6 (12%) | 19 (25%) | 0.09 |
| COPD** | 2 (4%) | 7 (9%) | 0.41 |
| Pacemaker/ICD | 7 (13%) | 14 (19%) | 0.37 |
| AF ablation therapy | 7 (13%) | 6 (8%) | 0.48 |
| LAA dimensions on CT: | |||
| Width | 30 (23–33) | 30 (22–32) | 0.42 |
| Length | 30 (25–40) | 30 (25–36) | 0.78 |
| Number of lobes | 1 (1–2) | 2 (1– 2) | 0.44 |
| Pre-procedure medication: | |||
| None | 3 (6%) | 0 | 0.10 |
| Antiplatelet agents | 7 (13%) | 10 (13%) | |
| VKA | 42 (81%) | 65 (87%) | |
| Indication for LAAC: | |||
| Stroke/TIA while on OAC | 4 (8%) | 21 (28%) | 0.001 |
| Failure/complication* while on OAC | 12 (23%) | 10 (10%) | |
| Contraindicated to OAC | 6 (27%) | 6 (6%) | |
| Labile INR | 22 (42%) | 38 (51%) | |
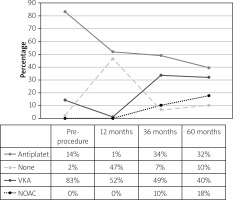
During the follow-up period, at 9–19 months, 48% of patients received no OAC, 52% received a VKA, and 0% received a NOAC; at 36–48 months, 41% of patients received no OAC, 49% received a VKA, and 10% received a NOAC; at 54–60 months, 42% of patients received no OAC, 40% received a VKA, and 18% received a NOAC, with no significant differences (Figure 1). Forty-seven percent of patients did not receive any anticoagulation treatment or antiplatelet agents during a 1-year follow-up.
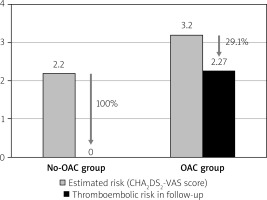
Thromboembolic risk reduction
There were no thromboembolic events in the No-OAC group. In the OAC group, thromboembolic events were observed in 3 patients (4%, p = 0.39). There was one episode of ischemic stroke after 12 months in a 58-year-old patient who was taking warfarin (CHA2DS2-VASc score = 6 and a history of previous stroke), one episode of TIA after 28 months in a 56- year-old patient also taking warfarin (CHA2DS2-VASc score = 2), and one episode of arterial peripheral embolism after 44 months in a 73-year-old patient who was taking acenocoumarol (CHA2DS2-VASc score = 2 and with deep vein thrombosis). The estimated thromboembolic risk reductions in the No-OAC and OAC groups are shown in Figure 2.
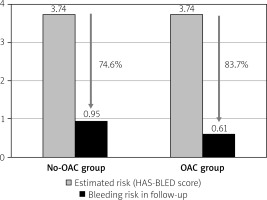
Bleeding risk reduction
In the follow-up period, there were 4 severe bleeding events: two hemorrhagic strokes occurred in the No-OAC group; in the OAC group, there was one hemorrhagic stroke in a 61-year-old patient who was taking acenocoumarol, and one gastrointestinal bleed in a 64-year-old patient taking dabigatran (p = 0.89). The estimated bleeding risk reductions in the No-OAC and OAC groups are presented in Figure 3.
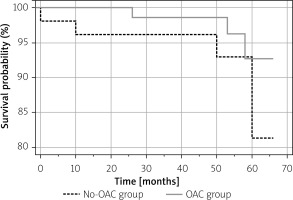
Mortality rate
The annual mortality rate was 1.9% in the No-OAC group (a total of 4 patients, including 2 cardiovascular deaths, 1 noncardiovascular death, and 1 death of unknown cause) and 0.93% in the OAC group (a total of 3 patients, 2 cardiovascular deaths, and 1 death of unknown cause). Kaplan-Meier survival analysis revealed no differences during the complete follow-up period (Figure 4).
Discussion
We present the first long-term outcome of oral anticoagulation therapy after a successful LAAO procedure. In our patients, postprocedure oral anticoagulation therapy (VKAs or NOACs) following the LAAO procedure with a LARIAT device did not reduce the risk of stroke, systemic thromboembolism, bleeding complications, or mortality during 5.5 years of follow-up.
Current ESC [2] and ACC/AHA/HRS [3] guidelines include a Class IA recommendation for oral anticoagulation in patients with atrial fibrillation (AF) with a history of a prior stroke or a CHA2DS2-VAS score ≥ 2. However, for cases in which OAC is contraindicated or ineffective, the LAAO procedure represents a feasible alternative, even in elderly patients [11]. In the last decade, the LAAO procedure for the management of patients with AF has been growing in popularity [8]. The ESC and AHA guidelines give the LAAO procedure a Class 2B recommendation for patients with atrial fibrillation in whom anticoagulation is contraindicated [2, 3]. However, at present, there are no universal guidelines for initiating post-LAAO anticoagulation [1, 17]. Because heterogeneous patient groups result in treatment variations, an analysis of the requirements for and the safety of ongoing LAAO treatment is paramount [1, 2].
LAAO procedures utilizing endocardial devices such as the Watchman or the Amulet have the highest risk of thromboembolic events in the first 6 weeks after the procedure [7, 8]. The LAA occlusion device may act as a thrombogenic focus in the absence of OAC or anticoagulation therapy in general [17]. Preclinical studies have shown complete epithelialization of the device surface by 45 days [18]. Because the prevention of acute device-related thrombosis is critical following the initial procedure, the PROTECT AF and PREVAIL trials used postprocedure treatment protocols of VKA anticoagulation therapy for 45 days, dual antiplatelet therapy for 6 months and aspirin thereafter [19, 20].
The LARIAT procedure uses a percutaneous approach to achieve suture closure of the LAA [12, 21–23]. In contrast to an endocardial system, epicardial LAAO exclusion with the LARIAT device leaves no foreign body, thereby minimizing the risk of postprocedure thrombus formation and a pericardial inflammatory response [18, 24]. However, histologic and anatomic analysis of the LAA after the LARIAT procedure suggests that a significant inflammatory response results in scarring and permanent closure of the LAA [22]. Therefore, our recommendation of life-long aspirin monotherapy differs from the standard protocols used following endocardial procedures [19, 20, 25]. Sievert et al. [26] demonstrated that patients with contraindications to OAC therapy undergoing LAAO required no postprocedure anticoagulation regimen to prevent embolic events. The majority of these patients did not receive any postprocedure antithrombotic agents. Although we recommended this standard regimen for our patients, the presence of comorbidities and physician preference meant that some patients continued to receive OAC. We observed that the proportion of patients receiving OAC changed over time: before the procedure, 84% of patients received OAC, and only 16% received antiplatelet agents. Fifty-two percent of patients received OAC 1 year after the procedure, 59% after 3 years, and 58% after 5 years. Notably, nearly 47% of patients received no anticoagulation treatment or antiplatelet agents during the follow-up.
There may be several reasons for the variations in anticoagulation therapy. The lack of ESC and AHA guidelines leaves physicians free to exercise their own discretion [2, 3]. Patient profiles also influence the choice; for instance, individual risks of thromboembolic events or hemorrhage should be taken into account. Special attention should also be paid to the patients with a history of a prior stroke, who had no contraindications to anticoagulation therapy and in whom left atrial appendage closure (LAAC) was performed because of the occurrence of a stroke/TIA while taking OAC. In contrast to ESC and AHA guidelines [2, 3], the Polish Cardiac Society expands LAAC indications to include patients with a history of cardioembolic stroke while taking OAC [1]. In our previous study, we demonstrated that patients with a history of a prior stroke may be the preferred group for LAAC, and LAAC may be preferred for all patients with a history of stroke/TIA regardless of the presence or absence of contraindications to anticoagulant therapy [27]. In the present study, continuation of anticoagulation therapy after LAAO was significantly higher in the subgroup of patients with a history of a previous stroke or TIA.
In addition, lack of familiarity with the relatively new LAAO procedure may make physicians more likely to continue OAC therapy. For example, we have observed situations in which physicians who have assumed the care of patients after an LAAO procedure insisted on using ongoing oral anticoagulation. Lastly, the addition of NOACs to the AF management guidelines partway through our follow-up period may have contributed to the observed rise in the number of patients receiving OAC [2, 3].
In our study, the baseline thromboembolic risk was the same between the two groups (including CHADS2 score, CHA2DS2-VAS score plus sex, age, heart failure, hypertension or previous stroke/TIA). We observed no thromboembolic events in the No-OAC group. In the OAC group there was one episode of stroke (a patient with a CHA2DS2-VAS score of 6 with a history of previous stroke), one episode of TIA (a patient with a CHA2DS2-VAS score of 2), and one episode of arterial peripheral embolism (a patient with a CHA2DS2-VAS score of 2 and deep vein thrombosis). There was no significant difference between these results.
In our opinion, there are also several others factors, apart from AF, that increase the risk of thromboembolism and that may have contributed to these thromboembolic events. However, these factors are not included in the CHA2DS2-VAS score and include obesity, diet, smoking, dyslipidemia, physical activity, metabolic syndrome, carotid artery disease, alcohol consumption, sickle cell disease or, as in our patient, deep vein thrombosis [28]. We believe that postprocedure OAC therapy had no influence on the risk of thromboembolic events due to AF after LAA elimination.
Surprisingly, the median HAS-BLED score was significantly higher in the OAC group than in the No-OAC group, although the reduction in the risk for bleeding was similar in both groups. Preprocedure anticoagulation was similar between the groups, with more than 80% receiving VKAs. However, half of the patients in the No-OAC group had an LAAO procedure because of either a contraindication to anticoagulation therapy or failure/complications resulting from anticoagulation treatments (50% of patients in the No-OAC group vs. 16% of patients in the OAC group). Therefore, due to the higher risk of bleeding in the No-OAC group because of the preexisting complications of anticoagulant therapy, the frequency of bleeding between the groups was similar.
Of note, the addition of NOAC to the management protocols may have influenced the HAS-BLED scores. A favorable risk-benefit profile and a lower risk of major bleeding have led to NOACs increasingly replacing VKAs in the treatment of AF. Of course, NOACs are not free of complications [29]. Nonetheless, we observed no differences in the bleeding rate between the groups.
In our study, postprocedure LAAO anticoagulation treatment did not impact the mortality rate. Based on the large real-life registries from the Watchman and Amulet report, the highest risk of bleeding is observed within 6 weeks following an LAAO procedure, in cases in which OAC is used until device endothelialization is complete. However, mortality after LAAO is more likely due to patients’ advanced age and considerable comorbidities rather than a result of bleeding complications [20, 21, 30].
Therefore, in our opinion, withholding oral anticoagulation treatment after the LAAO procedure with the LARIAT device is safe for patients after long-term follow-up.
This is a nonrandomized, retrospective, observational single-center study. The major limitations of our study include the estimation the overall value of LAAO, the lack of a control group, and the use of only a calculated stroke or bleeding risk score for analysis. In addition, the analyzed groups are small, and the results are underpowered. There were also no rigid criteria for postprocedure anticoagulation treatment, and the final decision was left to physician and patient preference. Lastly, we did not have information about whether the doses used in the postprocedure anticoagulation treatment were therapeutic. Because we analyzed only patients who underwent an LAAO procedure using the LARIAT, which is an epicardial device, our results cannot be easily extrapolated to patients undergoing other LAAO procedures.
Conclusions
Our long-term outcomes suggest that there is no need to administer oral anticoagulation after an LAAO procedure. When compared with patients receiving OAC, withholding OAC after an LAAO procedure in patients with AF is associated with similar (1) stroke prevention rates, (2) bleeding prevention rates, and (3) safety endpoints and long-term efficacy.