Summary
Data regarding the duration of dual antiplatelet therapy (DAPT) in patients with drug-eluting stent restenosis (DES-ISR) treated with percutaneous coronary intervention (PCI) and drug-eluting balloons (DEB) or DES are not unambiguous. In the current study we evaluated a total of 1,367 consecutive patients with DES-ISR, who underwent PCI with DEB or DES. Treatment with DAPT in patients with DES-ISR was related to better long-term outcomes in the case of PCI with DES than DEB, independently of DAPT duration. DAPT > 6 months is related to a higher stroke rate, independently of treatment type (DES and DEB), compared to DAPT ≤ 6 months.
Introduction
Dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) of in-stent restenosis (ISR) with either drug-eluting thin stents (DESs) or drug-eluting balloons (DEBs) is the treatment of choice in the majority of patients with obstructive coronary artery disease (CAD) [1]. The inclusion of DAPT and its duration are determined by a number of factors, including the presentation of CAD, the need for chronic anticoagulant therapy, the type of anticoagulant therapy, the risk of bleeding and ischaemic events. The situation is further complicated by PCI due to ISR. Then, in order to determine the type of DAPT treatment and its duration, we are usually obliged to take several additional factors into account, including, for example: following the procedure of restenosis (plain-old balloon angioplasty, PCI with DEB, DES or bare-metal stent (BMS)), whether it is the first or further restenosis, and in which artery the restenosis occurs. Based on the current guidelines proposed by the European Society of Cardiology (ESC), the use of DES or DEB is equally recommended in patients undergoing PCI of DES-ISR (class of recommendation I and level of evidence A), while recurrent diffuse ISR favours coronary artery bypass grafting (CABG) over PCI [1]. This is especially true in patients with diabetes, reduced left ventricle ejection fraction (LVEF), contraindication to DAPT and multi-vessel disease with SYNTAX score ≥ 23 points [1]. However, these guidelines do not precisely define the duration of DAPT treatment in patients undergoing PCI due to ISR, and 6 months of treatment are recommended in patients with chronic coronary syndrome (CCS) and 12 months in patients with acute coronary syndromes (ACS), further depending on the co-existence of modifying factors [1]. In patients treated with DEB, the use of which is recommended among individuals with ISR, DAPT treatment is recommended for 6 months. In patients with DES-ISR, treatment with PCI with DEB is classified on par with DES treatment [2, 3]. In a recent European consensus on platelet therapy, it was stated that there is a lack of data on the duration of DAPT treatment in patients following PCI with DEB [4], and the available data in patients from the PCI group with DEB due to ISR favour a duration of DAPT between 3 and 12 months [5–7]. However, in some studies, the length of DAPT is even indicated to be shorter than 1 month [8].
Aim
Herein, the aim of analysing the DEB-Dragon Registry was to investigate the relationship between clinical outcomes during follow-up in patients treated due to DES-ISR with DEB or DES stratified by the duration of DAPT.
Material and methods
Patients
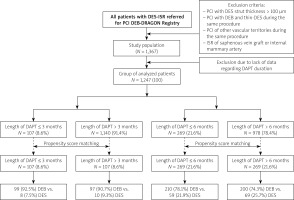
The DEB-Dragon Registry is a large multi-centre observational study conducted at 8 high-volume PCI centres. These data included all 1,367 consecutive patients with DES-ISR who met inclusion criteria while not meeting exclusion criteria, and were treated with either paclitaxel-DEB or thin-DES in the period from February 2008 until October 2019. Patients were divided according to the duration of DAPT (Figure 1). The division of patients was carried out according to the duration of DAPT: ≤ 3 months and > 3 months, and ≤ 6 months and > 6 months. Afterwards, patients were matched according to propensity score and we selected 107 patients treated with DAPT ≤ 3 months and 107 patients > 3 months, as well as 269 patients treated ≤ 3 months with DAPT and 269 > 6 months (Figure 1). Patients who demonstrated both types of PCI during the same procedure were excluded from the study. Since we aimed to investigate outcomes in native vessels, patients with ISR in the saphenous vein graft were also excluded from the study. Patients who had PCI of other vascular territories during the same procedure were additionally excluded. However, patients with repeated interventions on the target lesion, including those with multiple stent layers, were eligible. The following thin-strut stents were used: Resolute (Medtronic CardioVascular, Santa Rosa, CA, USA), Promus (Boston Scientific, Natick, MA, USA), Ultimaster (Terumo Corporation, Tokyo, Japan), Orsiro (Biotronik AG, Bülach, Switzerland), Alex (Balton, Warsaw, Poland), Synergy (Boston Scientific, Natick, MA, USA), Xience (Abbott Vascular Devices, Santa Clara, CA, USA). The paclitaxel-DEB types were as follows: Agent (Boston Scientific, Natick, MA, USA), Dior (Eurocor GmbH, Bonn, Germany), Elutax (Aachen Resonance GmbH, Aachen, Germany), Essential (iVascular, Barcelona, Spain), In.Pact Falcon (Medtronic Vascular, Santa Clara, CA, USA), Pantera Lux (Biotronik AG, Bülach, Switzerland), Restore DEB (Cardionovum GmbH, Bonn, Germany), SeQuent Please Neo (B. Braun Interventional Group, Ltd., Melsungen, Germany) [9]. Angiographic data of participants included in the study were collected and recorded in the central registry. Outcome data were obtained from the central database of the National Health Fund Service of the Ministry of Health, and no patient was lost to follow-up. The patients’ data were protected according to the requirements of Polish law, GDPR, and hospital Standard Operating Procedures (SOPs). The study was conducted in accordance with the Declaration of Helsinki and was registered at ClinicalTrials.gov, Identifier: NCT04415216. The exact methodology of the research was described in the first paper from the registry presented above [10].
Study end-points
The primary efficacy end-point was target lesion revascularisation (TLR). The secondary end-points were device-oriented adverse cardiac events (device oriented composite endpoints (DOCE), defined as a composite of cardiac death, TLR, and target vessel MI), target vessel revascularisation (TVR), myocardial infarction (MI), death and stroke.
Statistical analysis
Continuous variables were considered as means with standard deviations or medians with the 1st and 3rd quartiles when appropriate. Nominal variables were presented as counts and percentages. Normal distribution was verified by the Kolmogorov-Smirnov test. Groups were compared using the t-test for continuous variables or theχ2 test for nominal variables or their non-parametric equivalents when appropriate. Standardised differences were calculated for all baseline variables before and after matching. From all of the baseline/demographic characteristics, those with a p-value lower than 0.2 or standardised differences higher than 10% for differences across groups were included in the logistic regression model used in propensity score matching (PSM). PSM was performed using the nearest neighbour algorithm. The groups were considered balanced if standardised differences for each of the analysed baseline/demographic characteristics were lower than 10%. Models were constructed with procedural data as covariates with the purpose of adjustment. Statistical analysis was performed using R, version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria, 2019) with the following packages: ‘MatchIt’, version 3.0.2 and ‘lme4’, version 1.1-21.
Results
Baseline patients’ characteristics before propensity score matching
Patients treated with DAPT for a shorter period (≤ 3 months) were older, more often treated with insulin, more frequently experienced kidney failure, peripheral artery disease, atrial fibrillation and CCS, while they less often experienced unstable angina (UA) and non-ST segment elevation myocardial infarction (NSTEMI). They were also more frequently treated with oral anticoagulants and less often with ticagrelor. Patients treated with DAPT ≤ 6 months experienced kidney failure, atrial fibrillation and CCS more frequently, while they less often experienced UA and NSTEMI. They were also more often treated with oral anticoagulants and less frequently with ticagrelor. Other baseline clinical presentations, patient characteristics and pharmacotherapy according to the duration of DAPT before PSM are presented in Table I.
Table I
Baseline clinical presentation, patient characteristics and pharmacotherapy according to duration of dual antiplatelet therapy before propensity score matching
Procedural success expressed as patent coronary artery by the Thrombolysis in Myocardial Infarction (TMI) grade flow scale occurred less often among patients treated longer with DAPT (> 3 and > 6 months). Coronary angiography results, culprit lesion characteristics, procedural indices and selected biochemical parameters according to the duration of DAPT before PSM, are presented in Table II.
Table II
Coronary angiography, culprit lesion characteristics, procedural indices, selected biochemical indices and left ventricle ejection fraction according to dual antiplatelet therapy duration before propensity score matching
Baseline patients’ characteristics after propensity score matching
Selected baseline indices presented according to the duration of DAPT after PSM are presented in Table III.
Table III
Selected indices according to duration of DAPT after propensity score matching
Clinical outcomes according to the duration of DAPT and type of PCI in DEB, DES and overall group before PSM – univariable analysis
Significant relationships for the groups pre-specified above are presented in Table IV. Study endpoints with no significant relation to the duration of DAPT, type of PCI and analysed study endpoint have been removed from the table. There were no such significant relationships between the duration of DAPT, independent of the type of PCI for re-PCI, CABG and TLR-CABG.
Table IV
Predictors of clinical outcomes according to duration of DAPT before propensity score matching – univariable analysis
Pairwise contrast considering type of PCI (DES vs. DEB) and duration of DAPT (> 6 vs. ≤ 6 months) before propensity score matching
In these analyses, the following relations could be found:
Major adverse clinical events (MACE):
DOCE as an endpoint:
DES + DAPT ≤ 6 months (superior) vs. to DES + DAPT > 6 months (p = 0.02);
DES + DAPT ≤ 6 months (superior) vs. DEB + DAPT ≤ 6 months (p < 0.001);
DES + DAPT ≤ 6 months (superior) vs. DEB + DAPT > 6 months (p < 0.001);
DES + DAPT > 6 months (superior) vs. DEB + DAPT > 6 months (p < 0.001);
DEB + DAPT ≤ 6 months (superior) vs. DEB + DAPT > 6 months (p = 0.02).
TVR:
DES + DAPT ≤ 6 months (superior) vs. DEB + DAPT ≤ 6 months (p = 0.02);
DES + DAPT ≤ 6 months (superior) vs. DEB + DAPT > 6 months (p = 0.008);
DES + DAPT > 6 months (superior) vs. DEB + DAPT > 6 months (p = 0.02).
TLR:
Predictors of se lected clinical outcomes assessed before propensity score matching by multivariable analysis
Predictors of increased and decrease rate of selected clinical outcomes – DOCE, TVRs, TLRs, MIs, overall deaths and MACE – are presented in Figures 2 A–F.
Figure 2
Predictors of selected clinical outcomes assessed by multivariable analysis in overall group of patients included in analysis before propensity score matching. A – Device-oriented clinical outcomes (DOCE), B – target vessel revascularisation (TVR), C – myocardial infarction (MI), D – target lesion revascularisation (TLR), E – overall death rate, F – major adverse clinical events (MACE)
CI – confidence interval, Cx – circumflex artery, DAPT – dual antiplatelet therapy, DEB – drug-eluting balloon, DES – drug-eluting stent, NSTEMI – non-ST segment elevation myocardial infarction, PCI – percutaneous coronary intervention, TL – target lesion, TIMI – Thrombolysis In Myocardial Infarction.

Study endpoints after propensity score matching
Selected clinical outcomes stratified according to the specified DAPT durations before and after PSM are presented in Table IV.
Kaplan-Meier estimates of selected clinical outcomes before and after propensity score matching
Pre-PSM
The percentage of:
TVR-free survival was significantly lower in the group of patients with DAPT ≤ 3 months vs. > 3 months (p = 0.02);
acute MI-free survival was significantly lower in the group of patients with DAPT ≤ 6 months vs. > 6 months (p = 0.03);
cardiovascular death-free survival was significantly higher in the group of patients with DAPT ≤ 6 months vs. > 6 months (p = 0.04);
DOCE-free survival was significantly lower in the group of patients with DAPT ≤ 3 months vs. > 3 months (p = 0.049);
stroke-free survival was significantly lower in the group of patients with DAPT ≤ 6 months vs. > 6 months (p = 0.049).
There were no other significant differences between the selected clinical outcomes according to the duration of DAPT (≤ 3 vs. > 3 months and ≤ 6 vs. > 6 months).
After PSM
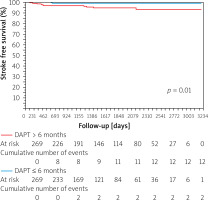
The percentage of stroke-free survival was significantly lower in the group of patients with DAPT ≤ 6 months vs. > 6 months (p = 0.01) (Figure 3). The mean time to stroke occurrence was 422.6 ±554.7 days and the median was 490 days with interquartile range: 290–1073 days. There were no other significant differences between the selected clinical outcomes according to the duration of DAPT (≤ 3 vs. > 3 months and ≤ 6 vs. > 6 months).
Discussion
The main findings of the current study are that patients treated for a shorter duration with DAPT (≤ 3 and ≤ 6 months) tend to be burdened more frequently with factors related to shorter treatment with DAPT, which included, for instance: older age, kidney failure or atrial fibrillation before PSM. Moreover, they were also more often admitted due to CCS but less ACS, and less frequently treated with newer generations of antiplatelet drugs. Univariate analysis performed before PSM demonstrated that patients treated for a longer period with DAPT (> 3 and > 6 months), independently of the group (DEB, DES and overall), presented poorer clinical outcomes. Considering pairwise analysis before PSM, it can be indirectly inferred that patients treated with PCI and DES have a better prognosis than those treated with DEB in terms of MACE, DOCE, TLR and TVR, independently of DAPT. PCI with DEB was found among risk factors of DOCE, TVR, TLR and MACE assessed by multivariable analysis before PSM in the overall group of patients treated due to DES-ISR. Furthermore, treatment with DAPT for longer than 6 months was related to a greater rate of MACE assessed by multivariable analysis before PSM in the overall group of patients treated due to DES-ISR. After PSM, patients treated with DAPT longer than 3 months had experienced greater TVR and DOCE, while patients treated with DAPT longer than 6 months had more strokes, TLR, TVR and DOCE. The Kaplan-Meier estimate, calculated in the overall group of patients treated due to DES-ISR, confirmed that patients treated with DAPT longer than 6 months had more strokes.
Analysing the results of the present study, there is quite a significant and natural difference in the characteristics of patients before PSM, which is a consequence of selecting DAPT duration. In the group of patients treated for a shorter period, we may observe a greater frequency of factors supporting a reduction in the duration of DAPT treatment, and these factors have been identified and used in everyday practice on many popular scales for assessing the risk of bleeding and ischaemic events in patients following PCI [11–14]. For example, the higher incidence of atrial fibrillation in the group of patients with a shorter duration of DAPT is associated with chronic anticoagulation, which theoretically may be associated with a lower risk of in-stent thrombosis, even after very early discontinuation of DAPT. This is noteworthy because, when analysing treatment outcomes before and after PSM, there are several indications that longer DAPT treatment is associated with poorer treatment outcomes, mainly in terms of greater frequency of MACE and DOCE, despite the fact that the final analysis using Kaplan-Meier survival curves only shows the greater incidence of strokes in the group of patients treated longer with DAPT. The availability of research results on the duration of DAPT used in the group of patients treated with PCI due to DES-ISR is somewhat limited. Therefore, we must also use the results of studies for the entire population of patients treated with PCI. An increased risk of bleeding with prolonged DAPT treatment (3 vs. 6 vs. 12 months) and ischaemic events with shortened DAPT treatment in patients with or without ACS undergoing PCI has been demonstrated in a number of publications [15]. A large part of the publications on the length of DAPT, especially those in which the results of PCI in patients with CCS or low-risk ACS are evaluated, provide neutral results, namely, no significant differences in the frequency of ischaemic and haemorrhagic complications depending on the duration of DAPT [16, 17]. However, for example, in a study published by Watanabe et al., it was demonstrated in a group of patients treated with PCI that 1 month of DAPT followed by clopidogrel monotherapy, compared to 12 months of DAPT with acetyl-salicylic acid and clopidogrel, was related to a significantly lower rate of cardiovascular and bleeding event composites, meeting the criteria for both non-inferiority and superiority [18]. Nonetheless, a meta-analysis is also available in which the duration of DAPT used in patients treated with PCI was analysed. The authors found that in patients undergoing implantation with newer-generation DES, long-term DAPT resulted in greater all-cause mortality than short-term DAPT [19]. There are a number of directions of the currently undertaken studies, one of them being the shortening of dual therapy instead of monotherapy conducted by new anti-platelet drugs, such as ticagrelor, in order to reduce the incidence of bleeding events [20]. Extended DAPT treatment with standard and reduced ticagrelor doses up to 3 years after PCI for ACS in patients at increased risk was also assessed, showing an increased benefit in terms of ischaemic events with an increased risk of non-fatal bleeding [21].
Nevertheless, the group of patients undergoing PCI due to DES-ISR is a very characteristic group of high-risk restenosis, which often, prior to PCI, is consulted within the “heart team” in order to make a choice that is better for a particular patient, i.e. percutaneous or surgical treatment. At present, the dominant belief is that each patient should be subjected to individual risk assessment of ischaemic and haemorrhagic events, considering various aspects, such as compliance, treatment price, life expectancy, planned surgical procedures, and patient preferences, which are not always in line with the current recommendations of guidelines and evidence-based medicine [22]. Additionally, each patient should undergo a detailed analysis of the surgical risk using the EuroSCORE II scale and the STS score [23, 24]. In order to determine the treatment method as well as the duration of DAPT treatment, one of the key elements seems to be the assessment regarding the complexity of lesions in the coronary arteries, and currently, the gold standard used for this purpose is the SYNTAX score [25].
The discussion on the relationship between the duration of platelet therapy depending on the type of treatment used (DES vs. DEB) is limited in the current analysis of DES-ISR by a number of factors, one of the main factors being the lack of precise information on the type of stroke. Moreover, currently available data concern the vast majority of patients who undergo PCI primarily within the native vessels, but not DES-ISR. A number of studies have demonstrated an increased incidence of stroke in patients with prolonged DAPT expressed as greater risk ratios of risk of stroke, including the ARCTIC-interruption, PRODIGY and ITALICS randomised controlled trials (RCTs) in different clinical presentations of angina [26–28]. Greater risk ratios of risk of stroke for shorter DAPT were found, among others in the CREDO and SECURITY RCTs [29–30]. No differences in risk ratios of risk of stroke were observed in the OPTIMIZE and RESET RCTs [16, 31]. When considering intracranial bleeding, the majority of RCTs published so far demonstrated that the risk ratio of risk of intracranial bleeding is greater in the case of longer than shorter treatment with DAPT independently of the clinical presentation of angina and type of second antiplatelet agent [28, 32–34]. Recently published studies performed on patients with stable angina and treated with PCI provided a net clinical benefit of anti-atherogenic over the pro-haemorrhagic effects of prolonged treatment with DAPT in selected groups of patients at increased cardiovascular risk, such as in diabetics, and with a history of PCI [35].
There are several limitations to this study. There was a lack of intravascular imaging data. Furthermore, we had no data on the mechanism of restenosis, quantitative findings such as reference vessel diameter, minimal lumen diameter or lesion length. While the sample size of this study was large, the research was not designed as a randomised trial but rather as an analysis of data from a retrospective registry, which has inherent limitations. In subsequent studies, patients should also be divided into those treated for CCS and those treated for ACS, separated by type of ACS. In the analysis, the risk associated with the complexity degree of changes in the coronary arteries expressed as the SYNTAX score was not taken into account. Another limitation in the correct interpretation of the study is the fact that in the follow-up period we do not have precise information on the type of stroke. Additionally, data on strokes were not prospectively collected. Moreover, similarly to other endpoints, strokes were not assessed by a blinded event committee. The adherence of participants to DAPT was also not assessed in the current study
Conclusions
The current analysis demonstrated that treatment with DAPT in patients with DES-ISR is related to better long-term outcomes in the case of PCI with DES than DEB, independently of DAPT duration. DAPT > 6 months is related to a higher stroke rate, independently of treatment type (DES and DEB), compared to DAPT ≤ 6 months. The duration of DAPT treatment in the group of patients treated with PCI due to ISR-DES should be selected individually, after assessing a number of factors not only related to the risk of bleeding and ischaemia.