Summary
The results of IFU-based feasibility of modern endovascular aneurysm repair (EVAR) in a range of 100 patients with asymptomatic abdominal aortic aneurysm (AAA) in the East-Central European population are presented. The overall EVAR feasibility reached 68%, with individual stent graft system applicability ranging from 20% to 65%. The most frequent causes of EVAR inapplicability were the wide diameter of the common iliac artery, its angulated proximal neck, and the diameter of its proximal neck out of range. Excluder Conformable seems to be the most applicable stent graft system. One-third of asymptomatic AAAs require an open repair or more advanced EVAR technology.
Introduction
Continuously increasing applicability of endovascular aneurysm repair (EVAR) due to the development of new devices and techniques of deployment and the evolution of manufacturers’ instructions for use (IFU) offer a possibility to treat abdominal aortic aneurysms (AAA) with complex anatomies. As a result, there are several commercially available stent graft systems that have different criteria for use, and also differ in regard to technical aspects of implantation. Since the clinical success of EVAR depends on proper patient and stent graft selection and expeditious stent graft implantation, it can be assumed that one has to limit their stent graft armamentarium to a minimum number of device types to gain sufficient proficiency in EVAR planning and performance. It is very important to apply EVAR within IFU also with regard to medico-legal issues. Though some EVAR feasibility studies have been published, they did not concern East-Central European populations, and some of them were based on the old generation of stent grafts, which are no longer in use and had a retrospective nature [1–6].
Aim
The aim of this study was to determine the applicability of currently registered and commercially available stent graft systems for endovascular treatment of AAAs in East-Central European populations.
Material and methods
Patients
Computed tomography angiograms (CTAs) of 100 consecutive eligible patients referred for the elective repair of an infrarenal AAA to the University Hospital’s Department of Vascular Surgery in the years 2013–2014 were analysed. The study complies with the Declaration of Helsinki, and ethical approval for this study was waived by the institutional bioethical committee because of the absence of signs of a medical experiment. All patients admitted to the hospital with a diagnosis of an infrarenal AAA were pre-screened. The inclusion criteria were the presence of an asymptomatic AAA of the maximum diameter ≥ 50 mm and good quality CTAs with a slice thickness of at least 0.625 mm comprising the entire abdominal aorta and the iliac and common femoral arteries. Patients with para- and suprarenal aneurysms, dissections, penetrating aortic ulcers, mycotic aneurysms, and previous aortoiliac interventions, with either suspected or diagnosed connective tissue disorders, were excluded from the study. Case enrolment was closed after recruiting 100 patients.
The suitability for the EVAR was specified based on a review of their CTA scans before the patients were qualified for either EVAR or an open surgical repair. The data on patients’ demographics and cardiovascular risk factors were collected.
Measures and definitions
The morphological analysis of anonymised CTAs was performed using the OsiriX DICOM (Digital Image and Communication in Medicine) viewer (Pixmeo Sàrl, Bernex, Switzerland) in the 3D multiplanar reconstruction mode. Diameter measurements were made orthogonally to the centreline of the artery from the outer wall to the outer wall for the outer diameter and from the inner wall to the inner wall for the inner diameter. The length measurements were made along the centreline. The proximal neck was defined as a segment of the non-aneurysmal aorta between the lower renal artery and the beginning of the aneurysm. The following morphological parameters were assessed: a) diameter, length, α and β angulation as well as presence and extent of thrombus and calcification of the proximal neck, b) diameter of the distal neck, c) maximum aneurysm length, and d) diameters and anatomy of the common (CIA), external (EIA), and internal iliac arteries (IIA). Factors such as the shape of the neck (conical) and iliac artery tortuosity were not taken into consideration because they were not mentioned in the IFU.
The feasibility assessment for EVAR was determined based on the latest available IFU of the analysed stent grafts (Table I), which were either FDA-approved or had a CE mark. Fourteen EVAR systems were analysed: AFX 2 Endovascular AAA System (Endologix, Irvine, CA), Altura Endograft System and Aorfix (Lombard Medical, Oxfordshire, United Kingdom), Anaconda (Vascutek, Inchinnan, United Kingdom), Endurant II Stent Graft System (Medtronic Cardiovascular, Santa Rosa, CA, USA), Excluder C3 and Excluder Conformable stent graft (W.L. Gore and Associates, Flagstaff, AZ, USA), E-vita and E-tegra abdominal stent graft (Jotec, Hechingen, Germany), Incraft stent graft system (Cordis Corporation, Bridgewater, NJ, USA), Ovation iX (Endologix, Santa Rosa, CA, USA), Treo Abdominal stent graft system (Bolton Medical Inc., Sunrise, FL, USA), Zenith Alfa and Zenith Flex (Cook Medical, Bloomington, IN, USA).
Table I
Instructions for use (IFU) of analyzed stent graft systems
| Stent graft type | Min. proximal neck diameter [mm] | Max. proximal neck diameter [mm] | Min. neck length [mm] | Max. angle β [°] | Max. angle α [°] | Min. diam. of distal neck [mm] | Min. diameter of CIA [mm] | Max. diameter of CIA [mm] | Min. length of distal landing zone of CIA [mm] | Min. diameter of EIA [mm] | Min. diameter of EIA2* [mm] | Min. aneurysm treatment length [mm] | Max. zone of calcification/ thrombus of the neck [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ovation iX1,3 | 16 | 30 | NA | 60° if neck length ≥ 10 mm 45° if neck length < 10 mm | NA | 21 | 8 | 25 | 10 | 5 | 4.5 | NA | 50/ND |
| AFX (2)2 | 18 | 32 | 15 | 60 | NA | 12 | 10 | 23 | 15 | 7 | 3.5 | NA | 50 |
| Treo2 | 17 16 | 32 if β < 60° 30 if β 60–75° | 10 if β < 60° 15 if β 60–75° | 60/75 | 45 | 21 | 8 | 13 20 | 10 15 | 6 if neck ∅ < 25 7 if neck ∅ 25–32 | 5 | NA | 50 |
| Incraft2 | 17 | 31 | 10 | 60 | NA | 18 | 7 | 22 | 15 | 5 | 4 | 128 | 50 |
| Zenith Flex2 | 18 | 32 | 15 | 60 | 45 | 20 | 7.5 | 20 | 10 | 7 if neck ∅ ≤ 22 7.7 if neck ∅ 23–28 8.5 if neck ∅ > 28 | 6 | NA | 50 |
| Zenith Alfa2 | 18 | 32 | 15 | 60 | 45 | 20 | 8 | 20 | 10 | 6 if neck ∅ ≤ 28 6.5 if neck ∅ > 28 | 6 | NA | 50 |
| Altura3 | 18 | 28 | 15 | 60 | NA | 20 | 8 | 18 | 15 | 5 | 5 | NA | 50 |
| Aorfix2 | 19 | 29 | 15 | 90 | NA | 20 | 9 | 19 | 15 | 7 | 7 | NA | 50 |
| Endurant II2 | 19 | 32 | 10 | 60 | NA | 20 | 8 | 25 | 15 | 6 if neck ∅ 19–25 7 if neck ∅ 26–32 | 5 if CIA ∅ 8–14 6 if CIA ∅ 15–25 | NA | 50 |
| Excluder C33 | 19 | 32 | 15 | 60 | NA | 18 | 8 | 25 | 10 | 6 | 5 | NA | 50 |
| Excluder Conformable3 | 16 | 32 | 10 if β < 60° 15 if β 60–90° | 60/90 | NA | 18 | 8 | 25 | 10 | 6 | 5 | NA | 50 |
| E-vita3 | 19 | 29 | 15 | 60 | NA | 20 | 11 | 23 | 15 | 7 | 6 | NA | 50 |
| E-tegra3 | 19 | 32 | 15 | 75 | NA | 20 | 8 | 25 | 15 | 6.5 if neck ∅ 27–32 6 if neck ∅ 19–26 | 5.5 | NA | 50 |
| Anaconda3 | 17.5 | 31 | 15 | 90 | NA | 20 | 8.5 | 21 | 20 | ≥ 6.6 if neck ∅ 21 ≥ 7.6 if neck ∅ > 21 | 6 | NA | 50 |
An AAA was considered suitable for EVAR with a particular stent graft system when the morphological characteristics fulfilled all the anatomical recommendations of the IFU. The IFU criteria of the analysed stent graft systems are presented in Table I.
Assuming that pooling two EVAR systems will increase the applicability of EVAR, all the analysed devices were combined in pairs, and the feasibility for EVAR for each pair was calculated.
Statistical analysis
The statistical analysis was performed with the GraphPad Prism software (version 6.04). Qualitative parameters were expressed as percentages, while continuous variables were reported as a median and quartiles. Qualitative parameters were compared using the χ2 test. The observed differences were considered statistically significant at p < 0.05.
Results
Characteristics of patients
Most of the study patients were male (82%), with a median of age of 69.5 (63–75) years. The median maximum diameter of AAA was 61.0 (55.0–69.5) mm and did not differ between men and women, 61.0 (55.0–69.0) mm and 62.5 (53.0–70.0) mm respectively (p = 0.656). Sixty-five percent of patients had hypertension, 44% had coronary artery disease, 34% had hyperlipidaemia, 25% had peripheral artery disease, 23% had diabetes, 7% had renal insufficiency (7%), 24% were obese (BMI > 30 kg/m2), and 20% were active smokers.
Morphological criteria for EVAR
Endovascular repair of the abdominal aortic aneurysm would be feasible in 68% of the study patients if the IFU were rigorously followed, and 32% were not suitable for any of the analysed stent grafts.
The largest number of patients, as many as 65%, were morphologically suitable for the Excluder Conformable stent graft, followed by Ovation iX and Endurant II, with the applicability rate of 51% and 47%, respectively. The most unsuitable stent grafts for the analysed group of patients were E-vita and Altura, with suitability of 20% and 34%, respectively (Figure 1). The differences in stent graft applicability were statistically significant (p < 0.001). The main limiting criteria within the analysed stent graft systems in order of prevalence are presented in Table II. Three patients demonstrated anatomic characteristics that made them suitable candidates for implantation of only one device: Excluder Conformable (2) and AFX (1).
Table II
Main limiting criteria in order of prevalence
Figure 1
Percentage of patients fitting the Instructions for use anatomical criteria of applicability of analyzed stent-graft systems
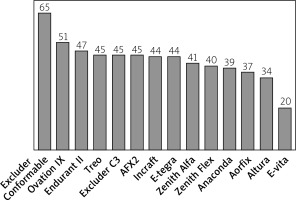
The maximal diameter of the CIA was the most prevalent cause of EVAR unsuitability that occurred depending on the stent graft system in 18–63% of patients, with Excluder and Ovation systems being the most and the E-vita system being the least applicable for wide CIAs. The CIA diameter was also the most common as a single limiting factor, which occurred in 9–28% of patients in 13 stent grafts. The proximal neck diameter out of range was the second most frequent cause of unsuitability, which occurred depending on the stent graft system in 5–27% of patients, with the Excluder Conformable system being the most and the E-vita system being the least applicable for out of range diameter of proximal neck; however, out of range proximal neck diameter was the single cause of unsuitability only in one device. The β angle was the third most prevalent cause of EVAR unsuitability, occurring in 6–12% of patients in 10 out of 14 evaluated stent grafts and a single cause of unsuitability in one device. Excluder Conformable, E-tegra, Anaconda, Aorfix, and Treo systems were the most applicable for a large β angle. The presence of > 50% thrombus of the proximal neck was found in 9% of patients. A short proximal neck resulted in unsuitability in 5 to 8% of the cases for all the devices, while a distal neck diameter was a limitation in 0–8% of the cases. The problem of the diameter of external iliac arteries was found minor in terms of suitability of the studied EVAR, except for Aorfix, where 11% of cases had an unsuitable EIA diameter. Major reasons of unsuitability for the evaluated EVAR systems are shown in Table II.
The pooled applicability exceeded the highest individual suitability rate for the pairs: Excluder Conformable – Treo by 2%, for Excluder Conformable – AFX 2 by 1%. The details are presented in Table III. Pairing Excluder Conformable with Treo made it possible to treat two more patients with a narrow proximal aortic neck and pairing the Excluder Conformable with AFX 2 made it possible to perform EVAR in one more patient with a narrow distal aortic neck.
Table III
EVAR applicability of paired stent grafts
Discussion
The present study aimed to evaluate the number of patients with asymptomatic AAAs who can be treated by EVAR with commercially available stent graft devices in conformity with the IFU. Although studies on EVAR suitability have been previously published, the present study has some unique features. A few former studies evaluated retrospectively the anatomical features in patients who had undergone endovascular repair procedures, or included only selected patients [2, 7, 8], whereas the present study analysed all patients with a diagnosed infrarenal AAA with the maximum aneurysm sac diameter of 50 mm or larger, regardless of the ultimate treatment plan, which resulted in a more representative group of AAA patients. Also, to the best of our knowledge, this is the first published study on the feasibility of the applicability of fourteen FDA-approved commercial devices among the East-Central European AAA population. Moreover, due to the constant accumulation of knowledge and the development of EVAR technology, the variety of commercially available EVAR devices is evolving. Most of the studies on the subject are more than 5 years old, and over that period of time, new devices have appeared, whereas some others disappeared from the market, so there is always a need to update the information on the EVAR applicability [1, 4, 7, 8].
The compliance with IFU criteria not only positively influences the long-term durability of EVAR but is also important from the medico-legal and ethical standpoint [7, 9]. The present study showed that 68% of the analysed patients met the IFU morphological criteria for at least one of the currently commercially available stent grafts. The most common barriers for using the selected stent graft were a wide diameter of the common iliac artery, a large β angle, and an inadequate diameter of the proximal neck. Proximal neck length, the diameter of the distal neck, and the presence of a moderate or severe thrombus of the proximal neck had a smaller influence on the stent graft suitability rate. Proximal neck calcification and α angulation and the diameter of the access vessels appear to play a minor role in the applicability of EVAR in the studied group.
In 20% to 40% of the patients treated with EVAR, the aneurysm extends to at least one and in 11–14% to both CIAs [3, 10, 11]. Based on the results from the current study, the diameter of the common iliac arteries constitutes a crucial criterion of whether a patient with AAA can be treated using EVAR. That is why EVAR systems such as Excluder, Endurant II, E-tegra, and Ovation iX, which within their standard configuration offer wide iliac extensions making it possible to treat patients with the CIA diameter up to 25 mm, demonstrated higher applicability in this study. The Zenith stent graft system offers a wide iliac extension as a custom-made device. That is why it was not taken into account in this study, although it may expand its real-life applicability. It has to be remembered, however, that the risk of late type IB endoleak is several times higher in patients treated with iliac limbs ≥ 20 mm as compared to patients treated with a distal iliac limb diameter < 20 mm [12]. To solve this therapeutic problem, some manufacturers have developed elements that allow the endograft to be extended into the external iliac artery, maintaining the perfusion of the internal iliac artery by a side branch that may further extend the applicability of EVAR [11].
In a study of 235 Greek patients with AAA, higher EVAR applicability rates than the ones observed in the present study were reported. The most applicable were Endurant II, Ovation iX, and Treo devices with the suitability rate of 80.7%, 78.9%, and 74.9%, respectively. The lowest feasibility rate was observed for Incraft, Aorfix, and Altura stent grafts with the suitability rates of 48.5%, 42.7%, and 34.5%, respectively. There were two criteria significantly limiting the suitability: the neck dimensions, and the CIA diameter. In that report, cases with extreme angulation, the presence of thrombus, or atherosclerosis of the CIAs had been excluded from their study, as they had been immediately considered unsuitable for endovascular repair [2]. Consequently, the cohort does not comprise a complete view of the variety of anatomies that would correspond to real-life clinical settings. The present work contains all infrarenal AAA patients, even those with adverse anatomies, which in the opinion of the authors gives a more reliable group representation and can partially explain the observed lower applicability rates. The differences in results of EVAR suitability may also be caused by the differences in aneurysm morphology associated with the patients’ region.
The results obtained in this study do not seem to reflect the real-life situation. In the first quarter of 2019, Endurant II was the most frequently implanted stent graft, with almost 44% of market share, followed by Excluder (23%) and Zenith (16.5%) [13]. However, in the present study, Endurant II is surpassed by two stent grafts: Excluder Conformable and Ovation iX. There may be several reasons for this discrepancy. Endurant II, in distinction to Excluder, has a suprarenal fixation. Since it does not change the IFU and does not influence the results, the type of proximal fixation should not influence the applicability. However, in the case of a suboptimal neck, many vascular surgeons prefer to use a device with suprarenal fixation. Endurant II is a successor of the Talent stent graft – they are both produced by the same company, Medtronic, present on the EVAR market for more than 30 years, enough time to have gained considerable trust among vascular surgeons. The same is true with regards to the Zenith and Excluder grafts, and it may explain the prevalence of these three systems over other grafts.
The data about the market share of the stent graft system come from the first quarter of the last year. At that time, Excluder Conformable, which has increased the applicability of the system in comparison to Excluder C3, was not available, which may also explain the prevalence of the Endurant II system. This fact partially explains the highest applicability of Endurant II in the aforementioned study comprising 235 patients from Greece [2]. In that study, however, Endurant II was better than the Treo, Ovation iX, and E-tegra systems, which may have resulted from different patients’ characteristics with regard to proximal neck morphology, more angulated necks in our patients, and shorter proximal necks in Greek patients. Furthermore, although it is not stated in the standard IFU, since 2016, Endurant II has been approved for use with chimney grafts, which may have further increased its applicability. Additionally, since September 2017, the IFU criteria of Endurant II have been extended to a short neck ≥ 4 mm, on condition that the Heli-FX EndoAnchor System fixation is applied. Because this approach requires additional devices to be used and, in our opinion, may not be durable, this indication was not included in the analysis. The data on the long-term results of that approach are still lacking [14, 15]. As a matter of fact, in the biggest registry reporting very good long-term results of infrarenal AAA treatment with Endurant, only 12% of patients had a proximal neck < 15 mm [16].
The Ovation iX grafts demonstrated a very high applicability rate both in the study from Greece and in the present one. Besides the possibility of treatment of patients with CIA ID up to 25 mm, it is unique in its proximal neck criteria that require a 16 to 30 mm ID only at 13 mm below the lower renal artery without specifying what the diameter above and below that level is. The seal at this level is ensured by a polymer filled ring. So far, its limited use in real life may result from low confidence in the durability of a proximal seal, especially after the disappointing result of the endovascular aneurysm sealing technology (EVAS) [17, 18]. However, the Ovation iX stent graft, in distinction to EVAS, also has an active suprarenal fixation system. And indeed, a recently published study of 1296 patients treated with that device, 50% of whom had complex aortic anatomies, demonstrated freedom from type IA endoleak in 95.8% and freedom from device-related reintervention of 92.4% over a period of 5 years [19]. A probable introduction of the Ovation Alto device in the near future with the sealing ring to be placed only 7 mm below the lower renal artery may further increase the applicability rate of this device [20].
It has to be noted, however, that the IFU criteria of currently commercially available stent grafts extend beyond what is considered the optimal anatomy for EVAR. On the one hand, it reflects the pursuit of the industry to cover a wide range of anatomies of AAAs. But on the other hand, one has to bear in mind that widening of anatomic criteria may compromise long-term results of EVAR. In a study that analysed the data of a large, multicentre cohort of 10,228 patients treated with EVAR, the independent morphological predictors of the AAA sac enlargement included an aortic neck diameter ≥ 28 mm, an aortic neck angle > 60°, and a common iliac artery diameter > 20 mm [9].
A bitter lesson was learned from the experience with Endovascular Aneurysm Sealing System (EVAS). According to its primary IFU, based on the analysis of 776 patients with AAA, more than 70% were considered suitable for EVAS [18]. Despite encouraging early reports, the device has been recalled due to poor long-term results, associated with type IA endoleak, graft migration, sac expansion, and the occurrence of an aorto-duodenal fistula [17, 18].
Pooling the morphological criteria of the most applicable device with any other only slightly increased the applicability of EVAR. So, it seems advisable that centres treating infrarenal AAAs should limit their armamentarium to two or three systems in order to gain maximum knowledge and expertise in device sizing, implantation techniques, and results without compromising the overall applicability of EVAR.
Conclusions
Within the East-Central European AAA population, the suitability rates for the analysed devices vary widely from 65% up to 20%. CIA diameters, as well as out of range and angulated proximal neck, are the morphological parameters significantly influencing the suitability rate of the evaluated stent grafts. A combination of the most applicable stent graft with any other device increases the applicability of EVAR only slightly. Thus, more advanced endovascular technologies, as well as open surgical repair techniques, are required to be able to treat all ranges of the anatomy of infrarenal AAA.








