Introduction
Acute coronary syndrome (ACS) remains the leading cause of death worldwide, despite significant efforts in primary and secondary prevention and invasive treatment with percutaneous coronary intervention (PCI) [1]. Approximately 5% to 25% of patients presenting with ACS have normal or near-normal coronary arteries on angiography (stenosis severity < 50%). The working diagnosis in these cases is myocardial infarction with non-obstructive coronary arteries (MINOCA) [2, 3]. However, MINOCA requires identification of underlying causes to optimize the treatment, improve prognosis, and promote the prevention of recurrent myocardial infarction. For this purpose, an accurate evaluation of the patient’s history, symptoms, and the use of non-invasive and invasive imaging is obligatory. Intracoronary imaging is a crucial diagnostic tool used in the process of identifying epicardial causes of MINOCA, especially those not visible in the coronary angiographies, such as plaque disruption, coronary spasm, coronary dissection, or thromboembolism [4].
Optical coherence tomography (OCT) and intravascular ultrasound (IVUS) have proven to be indispensable methods that allow a thorough assessment of the coronary arteries in acute and chronic coronary syndromes. OCT may precisely identify the culprit lesion or exclude other mechanisms, such as coronary dissection or in situ thrombosis, that may not have been adequately assessed during angiography [5, 6].
The main objective of this review is to demonstrate the usefulness of OCT in determining the atherosclerotic mechanism of MINOCA and thus the choice of the treatment strategy.
Fundamentals: optical coherence tomography
Intracoronary imaging (ICI) overcomes the limitations of coronary angiography, allowing both the lumen and the vessel wall to be visualized and assessed. ICI is used during diagnostic angiography to evaluate the severity of the lesion and evaluate the composition of the atherosclerotic plaque, which translates into the decision regarding revascularization and planning of the treatment strategy [7]. Currently, two methods of intracoronary imaging are used in everyday practice, i.e., IVUS and OCT, which both provide cross-sectional images of the vessel wall with a high resolution [8, 9]. Table I compares the basic technical parameters and the lesion characteristics in the OCT and IVUS examinations.
Table I
Basic differences between OCT and IVUS, including technical and pathological characterization of coronary lesions
IVUS enables imaging of the cross-sectional anatomy of the coronary artery wall in real time with 70 to 200 μm axial resolution, 200 to 400 μm lateral resolution, and 4 to 10 mm penetration depth. The images are obtained by transmission and reception of high-frequency ultrasonic waves by a mechanically scanning IVUS transducer (20–40 MHz) or a radial array transducer (10–20 MHz) [8, 10].
OCT uses near-infrared light to obtain high-resolution cross-sectional images of the coronary artery. The images are formed by measuring the echo time delay and the intensity of the backscattered optical signals from various tissue interfaces of the vessel wall. OCT uses interferometric detection to compare the signals reflected off the tissue with a fixed reference signal. The very high frequency of the light provides a 10–15 µm axial resolution of intravascular pathology, which is significantly higher than that of ultrasound, although reducing tissue penetration to 1–2 mm [11, 12]. There are two types of OCT systems: historical time-domain OCT (TD-OCT) and the currently used Fourier-domain OCT systems, also referred to as frequency-domain OCT (FD-OCT) or optical frequency domain imaging (OFDI). FD-OCT systems can measure all echo time delays that form an A-line at much higher imaging speeds allowing significantly faster image acquisition rates than the original TD-OCT systems [5].
OCT for diagnosing patients presenting with MINOCA and suspected epicardial causes
Among available modalities, OCT provides the highest spatial resolution allowing detailed visualization of plaque pathology in individuals with MINOCA. In a study by Gerbaud et al., which included 40 patients with MINOCA, OCT identified a potential culprit in 80% of patients, including 35% plaque rupture, 30% plaque erosion, 7.5% solitary thrombus, 5% SCAD and 2, 5% calcified nodule [13]. The authors emphasize that the most significant diagnostic benefit applies to the combination of both methods, OCT and magnetic resonance imaging (MRI). Reynolds et al. presented the largest study to date, involving 145 women diagnosed with MINOCA [14]. All patients had undergone optical coherence tomography, in 59.3% of cases three-vessel OCT. CMR was analyzed in 116 patients. In the OCT, a possible culprit lesion was identified in 46.2% of patients, including plaque rupture, intra-plaque cavity, or a layered plaque phenotype in 39% of patients, thrombus without plaque rupture in 3.5% of patients, and 1 case of SCAD. The authors confirmed previous findings that combined OCT and CMR allowed the identification of the cause of MINOCA in the vast majority of patients (84.5%). One of the interesting findings of this study was that 40% of patients with normal CMR had an OCT-identified culprit lesion. These data further emphasize the importance of OCT imaging in diagnosing MINOCA causes. The main limitations in detecting the coronary cause of MINOCA are the low number of patients with three-vessel OCT and the delay in imaging.
Taruya et al. revealed high-risk lesions in 51% of ACS patients with MINOCA, including several different substrates in the culprit artery, such as ruptured plaque (15.9%), calcified nodule (11.0%), spontaneous coronary artery dissection (8.5%), lone thrombus (8.5%), thin-cap fibroatheroma (6.1%), and plaque erosion (1.2%) [15]. Opolski et al. found plaque disruption and coronary thrombus in 24% and 18% of patients, respectively [16]. In addition, the infarct-related artery (IRA) showed a significantly higher frequency of plaque disruption, thrombus, thin-cap fibroatheroma, and fewer cholesterol crystals than the non-IRA. Similarly, coronary vessels supplying the infarct-related territory presented a higher frequency of plaque disruption, thrombus, and thin-cap fibroatheroma than coronary vessels in the non-infarct territory.
Case presentation
We present a case of a 29-year-old man with hypercholesterolemia who was admitted to our hospital for several days of recurrent chest pain. Two months ago, the patient was hospitalized in another center due to a non-ST segment myocardial infarction. Coronary angiography revealed normal coronary arteries. The patient was discharged from the hospital without an additional diagnosis of the causes of MI and no pharmacological treatment.
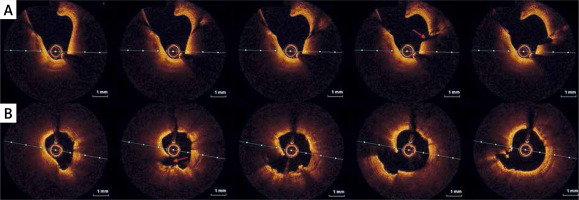
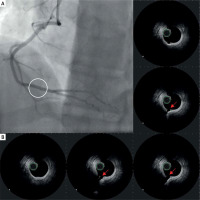
During the current hospitalization, coronary angiography with intracoronary OCT imaging was performed (Figure 1 A). Diffused atherosclerosis with a ruptured plaque in the right coronary artery (RCA) was identified (Figure 1 B). The patient was qualified for intensive conservative treatment. In addition, MRI confirmed the post-infarction scar of the entire thickness of the inferior wall with normal global systolic function. Pharmacological treatment, i.e., dual antiplatelet therapy (DAPT) for 12 months, β-blocker, and statin in the highest available dose, was included. After 3 months of follow-up, the patient remained free from angina and cardiovascular incidents.
Figure 1
A – Angiographic view of right coronary artery (RCA). Angiographically normal coronary artery. B – IVUS images of raptured plaque in the distal segment of RCA
As presented above, OCT is an excellent tool in determining the atherosclerotic etiology of MINOCA [14, 17]. However, the delay from the onset of chest pain to the performance of OCT is said to affect its diagnostic value. Therefore, OCT should be performed as soon as possible to evaluate appropriately for any substrate.
Plaque rupture
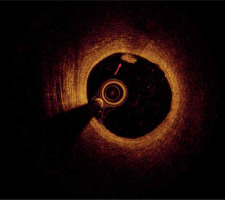
As mentioned before, the plaque rupture might be the potential coronary artery lesion responsible for MINOCA, as well as the most frequent mechanism of coronary thrombosis [14]. It can be identified by OCT as a fibroatheroma with fibrous cap disruption over the necrotic core, with or without cavity formation (Figure 2) [18]. When the necrotic core comes into contact with the bloodstream, the thrombus is formed. A typical sign of plaque rupture is the presence of flaps, which are left over from a tear of a fibrous cap that protrudes into the vessel lumen. In OCT, flaps appear bright and relatively homogeneous. The rupture creates a connection between the vessel lumen and the plaque lumen, which often remains empty, diffusely delineated, and appears dark.
Plaque erosion
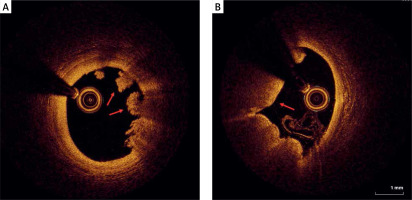
Another possible finding in the vessels is plaque erosion, defined as the presence of irregularly delineated thrombus masses located against the vessel wall that extend into the lumen without plaque rupture being visible in more than one adjacent cross-section (Figure 3). The thrombus appears non-homogeneous and is relatively bright due to the stronger reflection (white thrombus).
An eruptive calcific nodule
On the other hand, calcified nodules are a less common cause of coronary thrombosis and are characterized by thrombus formation over a nodular calcification protruding into the lumen causing disruption or injury of the thin fibrous cap (Figure 4). Thrombosis of calcified nodules can be identified as evidence of a signal-poor heterogeneous thrombus in conjunction with calcium protruding into the lumen and with sharp borders and low signal attenuation. Superficial calcifications (Figure 5) are independently associated with plaque rupture and intraplaque hemorrhage, probably due to the asymmetrical distribution of biological stress in plaques.
Figure 4
Eruptive calcific nodules (OCT). A, B – Nodular calcification protruding into the lumen through a disrupted thin fibrous cap (asterisk)
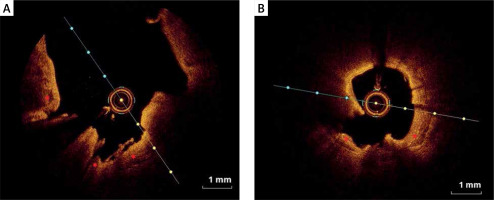
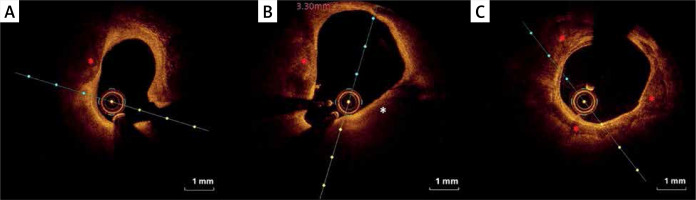
Figure 5
Calcified plaques (OCT). A – Spotty calcium deposits (red asterisk) defined as a deposit with length < 4 mm and maximal arc < 90°. B – Spotty calcium deposits (red asterisk) and thin-cap fibroatheroma (TCFA) (white asterisk). C – Large calcium deposits (red asterisk) defined as length ≥ 4 mm or maximal arc ≥ 90°
A lone thrombus
In some rare cases, it is impossible to visualize the ruptured atheroma, whereas it is feasible to picture a lone thrombus, defined as an intracoronary mass attached to the artery wall or floating within the lumen with high (red thrombus) or low (white thrombus) signal attenuation. A white thrombus is characterized by high signal intensity, weak absorption, and weak shadowing (Figure 6 A). It has diffused walls and heterogeneous structure, primarily due to incomplete fibrin polymerization. A red thrombus appears as an irregular mass with high signal intensity and weak shadowing (Figure 6 B). It has smooth walls and homogeneous areas due to an organized thrombus structure.
Spontaneous coronary artery dissection (SCAD)
SCAD is mainly diagnosed on the basis of angiographic assessment. However, the evaluation of SCAD type 2 in the angiographic image can often be ambiguous and similar to atherosclerotic lesions, hence the recommendation for the use of intracoronary imaging (OCT and IVUS). Intravascular imaging enables one to visualize the dissection with an intravascular hematoma or false lumen, without the presence of sclerosis.
Even though recent evidence suggests that OCT can be safely performed in patients with SCAD, IVUS appears to be more suitable than OCT [19, 20], as there is no necessity to administer contrast, and therefore the potential risk of further dissection propagation due to high-pressure injection of contrast is minimized. Furthermore, the excellent penetration of IVUS ensures visualization of the entire vessel wall, particularly in the case of deep dissection affecting the media.
Nevertheless, the unquestionable advantage of OCT is high resolution, which enables a detailed demonstration of the longitudinal extent and location of the intramural hematoma with the distal exit of the dissection and the intimal flap [21].
The use of OCT for PCI in SCAD can prevent over- or under-coverage of the stent and avoid premature sealing of the intimal flap and expansion of the hematoma. OCT can be a valuable method of coronary imaging to guide PCI when SCAD lesions are suspected.
OCT detection of high-risk lesions and prognosis of patients with MINOCA
Recent studies revealed that the prognosis of ACS with non-obstructive CAD includes a high risk of cardiovascular mortality and morbidity [22]. Moreover, patients with MINOCA are less likely to receive optimal secondary prevention treatment (e.g., aspirin, statins) than patients with obstructive CAD [23]. In this regard, the OCT diagnosis of high-risk lesions as an underlying cause of MINOCA may have therapeutic implications, including optimal pharmacological treatment and invasive treatment in selected patients [2]. Nevertheless, there is still a lack of conclusive studies that would define the coronary lesion characteristics associated with a worse prognosis. Below, we discuss the available data on the impact of coronary lesions found in OCT on ACS prediction with non-obstructive CAD.
Taruya et al. categorized lesions according to their morphology into high-risk (HL) and non-high-risk lesions (NHL) [15]. For HL, he included: ruptured plaque, calcified nodule, lone thrombus, thin-cap fibroatheroma (TCFA: minimal cap thickness < 70 µm), spontaneous coronary artery dissection (SCAD), and plaque erosion, identified based on the OCT consensus. Lone thrombus was diagnosed when the lesions met the following criteria: (i) the thrombus was attached to the normal arterial wall or intact fibrous-cap plaque, (ii) there was no apparent deficit in the arterial wall surface area, and (iii) there was no high-risk lesion in the proximal site. The frequency of recurrent ACS events was significantly higher in the HL group than in the NHL group, 4 (10%) vs. 0 (0%) patients. All of them occurred at the segment where the HL was previously identified. The Kaplan-Meier curve showed a worse prognosis in the HL group than in the NHL group (p = 0.040) [14]. In addition, patients with a better response to anti-thrombotic therapy in terms of thrombus reduction from baseline to 1 month as assessed by OCT required stent implantation less frequently over the next 4 years.
Opolski et al. also compared the results of OCT and cardiac magnetic resonance (CMR) in patients presenting with MINOCA [16]. Plaque rupture and thrombus were more frequently found in OCT in individuals with ischemic myocardial injury in CMR. In their study, an immediate interpretation of OCT resulted in a modification of the initial treatment strategy in 16% of patients.
In the SWEDEHEART registry subanalysis, 9,092 patients with MINOCA were identified [24]. Recurrent MI occurred in 570 (6.3%) patients approximately 1.5 years after the index event. The study showed a poor prognosis of all-cause death in patients with prior MINOCA re-admitted for new MI. About 22% of MINOCA patients with recurrent infarction died during follow-up, and half of the deaths were cardiovascular. The unfavorable prognosis was most pronounced in patients enrolled in a conservative, non-invasive strategy.
Discussion
Both intracoronary imaging modalities, IVUS and OCT, are essential tools for the pre-PCI evaluation of the coronary artery and post-PCI stent assessment and procedure optimization. However, due to the different technical parameters, it is preferable to use one of them in some conditions. As shown above, in the diagnosis of myocardial infarction with non-obstructive coronary arteries, optical coherence tomography is the preferred modality. The high resolution of OCT allows for accurate characterization of the coronary lesions, including the diagnosis of unstable and ruptured plaque and other pathologies responsible for MINOCA, such as coronary dissection or intramural hematoma.
OCT better distinguishes between various types of atherosclerotic plaques and lesion characteristics compared to IVUS. Moreover, only OCT enables the measurement of calcification thickness, which is crucial for the treatment strategy, including the use of atherectomy. OCT, unlike IVUS, allows evaluation of the vessel behind the calcification (without shadowing), which is essential for the correct sizing of the vessel. However, OCT penetration may be a limitation in the imaging of deeply localized calcifications in a significantly remodeled vessel [25]. In turn, in ostial left-main lesion assessment and guidance, IVUS should be considered, as blood clearance needed for OCT may be challenging, if not impossible [26]. Both methods are appropriate for pre-PCI stent sizing and identifying the exact deployment site; however, due to the higher resolution, OCT may result in a more accurate size and landing zone, which is especially important in complex lesions and lesions with uncertain morphology, including calcifications [27].
Similarly, OCT provides more detailed insight into the post-PCI stent deployment (apposition, expansion) and possible complications (edge dissection). In addition, mechanisms associated with stent failure (thrombosis, in-stent restenosis, malapposition, under-expansion) may be identified with greater sensitivity in OCT. It should also be remembered that in patients with renal insufficiency, only IVUS should be considered, which may additionally reduce the amount of contrast used.
Importantly, recent studies have shown that MINOCA patients have a 1-year mortality and rehospitalization rate similar to those with MI with obstructive coronary artery disease (MI-CAD) [28–32]. Choo et al. reported a similar incidence of all-cause death, cardiac death, noncardiac death, and reinfarction between MINOCA and MI-CAD [32]. The only difference was in the frequency of repeat revascularization, which was significantly lower in patients with MINOCA (1.3% vs. 7.2%; HR = 0.17; 95% CI: 0.07–0.41; p < 0.001). It was also found that the incidence of angina symptoms at follow-up was comparable in the two groups, significantly affecting the quality of life, healthcare and socioeconomic-related costs [29].
According to Rakowski et al., in 2016, 49,893 patients with non-ST-segment elevation (NSTEMI) or ST-segment elevation (STEMI) myocardial infarction entered the ORPKI registry. MINOCA was defined as a non-obstructive coronary artery disease (CAD) and a lack of previous coronary revascularization. MINOCA was identified in 3924 (7.8%) patients and the clinical presentation was more often NSTEMI than STEMI (MINOCA: 78 vs. 22%; obstructive CAD 51.1 vs. 48.9%; p < 0.0001). MINOCA patients were younger and more often female, with significantly lower rates of diabetes, smoking, arterial hypertension, kidney disease, previous MI and previous stroke compared to patients with obstructive CAD [33].
Considering that MINOCA patients are younger and have a lower frequency of cardiovascular risk factors than those with MI-CAD, the question arises why the prognosis of MINOCA patients is so poor. Undoubtedly, one reason is sub-optimal pharmacological treatment, especially with a lower percentage of antiplatelet drugs and statins [34]. The question remains whether we should also modify our approach to invasive treatment. In previous studies, patients with MINOCA treated with percutaneous coronary angioplasty were excluded from further follow-up and analysis. Consequently, data comparing the efficacy and safety of the conservative versus invasive strategy in treating patients presenting with MINOCA are still lacking. The EROSION (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion) study demonstrated that patients with ACS caused by plaque erosion might be safely treated with aspirin and ticagrelor without stenting for ≤ 1 month [35]. Moreover, 1-year follow-up revealed that most (92.5%) remained free of a major adverse cardiovascular event for ≤ 1 year, and the median residual thrombus volume decreased significantly from 1 month to 1 year [36]. Almost half of the patients (46.9%) had no residual thrombus at 1 year.
Only a few observational studies and case reports provide data on the safety of conservative treatment of ruptured plaque, which is the most common coronary finding in MINOCA. Thus, in the present state of knowledge, the primary role of OCT in MINOCA patients is to identify the coronary cause of MI. Optimizing pharmacological treatment in this group of patients, especially dual antiplatelet therapy and statins, improves the prognosis by reducing the risk of recurrent cardiovascular events. Despite insufficient data to confirm the advantage of an invasive treatment strategy, OCT enables the selection of lesions such as ruptured plaque, calcified nodule, lone thrombus, TCFA, SCAD, and plaque erosion, the occurrence of which is associated with a significantly higher frequency of recurrent ACS events. Thus, it seems rational to consider an invasive strategy in patients with high-risk coronary artery lesions and specific clinical conditions. Clinical indications include recurrent angina, ventricular arrhythmias, dynamic changes in the ST-T segment on ECG, or hemodynamic instability.
MINOCA should be considered as a heterogeneous syndrome, requiring comprehensive multimodal diagnostics to elucidate potential underlying mechanisms. Reducing the severity of angina and preventing rehospitalization to reduce healthcare costs and improve quality of life and long-term prognosis are priorities in this patient population. In the diagnostic process, MRI of the heart plays a crucial role, as it enables one to confirm myocarditis, as well as other rare causes of MINOCA, such as takotsubo cardiomyopathy. Nevertheless, the detection of ischemic lesions in the myocardium is highly suggestive of coronary artery disease and justifies the necessity to perform intravascular imaging. OCT provides detailed cross-sectional images with very high resolution enabling confirmation of the ischemic origin of MINOCA and implementation of intensive pharmacotherapy of MI, which directly improves the prognosis of patients with MINOCA. Undoubtedly, it is necessary to further investigate the importance of OCT in order to establish the proper treatment strategy of MINOCA, i.e., conservative vs. invasive, especially in the presence of high-risk lesions visualized in OCT.