Purpose
The American National Cancer Institute SEER program reports that 12.5% of men will be diagnosed with prostate cancer during their lifetime, with this being responsible for 10.7% of all male cancer mortality in 2022 [1]. Prostate brachytherapy (BT) is a cancer treatment, which delivers a radiation dose to the target volume while sparing surrounding organs at risk [2]. Unfortunately, a proportion of men are not directly considered eligible for BT, as access to the antero-lateral part of the prostate can be obstructed by the pubic arch in conventional treatment approaches utilizing parallel running, straight implant needles. This is referred to as ‘pubic arch interference’ (PAI). Limitations in positioning low-dose-rate (LDR) sources or high-dose-rate (HDR) source dwells behind the pubic arch, may prevent effective irradiation, as an adequate implant geometry in the target volume cannot be obtained.
The American Brachytherapy Society (ABS) guidelines state that a prostate volume (Vp) of > 60 cc is technically more challenging as PAI is more prevalent with enlarged prostates, and such a Vp is a relative contraindication for BT [3]. Earlier studies reported that 9% to 38% of patients had a Vp ≥ 60 cc [4-7]. However, the relation between Vp and the occurrence of PAI is not strong, and large prostates have been successfully implanted with good results for both dosimetry and biochemical control without excessive toxicity [2, 5, 7]. Therefore, the Groupe Européen de Curiethérapie and the European Society for Radiotherapy & Oncology (GEC-ESTRO) Advisory Committee for Radiation Oncology Practice (ACROP), have recently adapted their guidelines related to gland size, which now state that a prostate volume of > 50-60 cc is no longer a contraindication for BT if there is minimal PAI [2]. A threshold of 10 mm overlap by the pubic arch is used to indicate minimal PAI on magnetic resonance imaging (MRI) or computed tomography (CT) scans with the patient in supine position, and for minimizing the incidence of PAI during the procedure with the patient in lithotomy position [4, 8]. For PAI in the lithotomy position, a threshold of 5 mm has been reported [8, 9].
The change in body position causes an anatomical rotation of the pelvis and prostate, estimated at 15 degrees by Strang et al. [10], with associated improved accessibility of the prostate [11]. However, the relation between change in position corresponding obstruction is patient-specific, and earlier studies showed that a significant proportion of patients with enlarged prostates had excessive PAI of ≥ 10 mm. Zheng et al. [12] reported that 10 of 40 patients with a mean Vp of 64 cc had PAI up to 15.1 mm on MRI scans, while Bellon et al. [4] showed that 3 of 9 patients with a Vp > 60 cc had 10 to 20 mm PAI on CT scans. Moreover, Wang et al. observed PAI up to 13 mm on CT scans in 71% of 21 patients with a prostate volume > 50 cc [6]. In addition to the prevalence of PAI in enlarged prostates, smaller prostate glands can be difficult to access if the pubic arch is narrow, observed in 5% to 25% of patients with a Vp < 60 cc [8, 13, 14].
In practice, attempts to correct minimal PAI can be made by implanting sources in a different position to that planned, or manipulating the ultrasound probe [9, 15], while excessive PAI demands more drastic solutions. The patient can be placed in extended lithotomy position [4, 11, 15], but this increases the risk for rectal needle penetration [16], and can result in movement of the prostate because of probe angulation or inflation [17, 18]. Furthermore, not all patients are able to be manipulated into the required body positions [16]. Free-hand oblique needle implantations without the use of transperineal template are difficult, and require experience [19, 20]. These solutions demand adjustments to the clinical set-up and workflow, and can result in an inadequate dose coverage [18]. Another solution to overcome PAI is downsizing the prostate with the use of hormonal therapy, such as androgen deprivation therapy (ADT) [8, 15]. ADT decreases the prostate volume by 25% to 40% in 3 months, but is associated with significant costs, prolonged treatment time, and morbidity [21, 22]. Occasionally, excessive PAI is encountered at the time of implantation. The planned implant geometry and dosimetry requirements cannot be met, and another subsequent curative treatment is required, such as external beam radiotherapy [9]. This introduces additional costs for the healthcare system and a considerable anxiety for the patient.
Steerable needles that enable circumventing of interference from the pubic arch may facilitate proper distribution of the source positions without a change in clinical set-up, and thereby potentially ensure adequate irradiation of the target volume [23]. Such needles allow for increased flexibility in needle placement, and may extend treatment options for patients with enlarged and obstructed prostates. To investigate the potential benefits of steerable needles, the present work evaluated PAI in 27 patients. Through simulation of rotations of the pelvis and prostate, we showed the extent, to which steerable needles can be used in patients with enlarged prostates and observed PAI.
Material and methods
Patients
Datasets of 27 anonymized patients were included. All patients had undergone a diagnostic MRI scan (MAGNETOM Aera 1.5T, Siemens Healthineers, Erlangen, Germany) in a supine position. Patient’s eligibility criteria for this study included clinical stage T1-T3b cancer, a Gleason score of ≥ 3+3, any serum prostate-specific antigen (PSA), and an original Vp of ≥ 60 cc based on the formula: Vp = π/6 × (height × width × length). This choice excluded small prostates as the literature reports that the potential of PAI is lower in a smaller Vp [4].
Segmentation
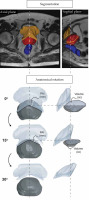
The prostate, pubic arch, urethra, and rectum were segmented manually by an experienced medical physics technician approved for procedure by a doctor (Figure 1A). The urethra was not always entirely visible on MRI; therefore, the best estimate was made based on any visible parts and clinical experience. The pubic arch was contoured as a single structure. Where the pubic arch separates, a slither was added to the contour to connect the left and right sides.
Fig. 1
Pipeline for the evaluation of the prostate datasets. A) Segmentation of the prostate, urethra, pubic arch, and rectum for all datasets. B) Anatomical rotation of the prostate and the pubic arch over six angles: 5°, 10°, 15°, 20°, 25°, and 30°. In sagittal plane, the pubic arch and the obstructed volume of the prostate are shown
Anatomical rotation
All MRI datasets were loaded into 3D Slicer (http://www.slicer.org/) and SolidWorks (Dassault Systèmes, SOLIDWORKS Corp.) for image processing. The pubic arch was outlined in the axial reference plane of the prostate and the accessible part of the prostate was subtracted from the whole gland to evaluate the obstructed volume and the maximum interference in supine position. A relationship between PAI and Vp was evaluated. To compare supine and lithotomy positions, PAI was quantified after six upward rotations of the anatomy in 5 degree steps, ranging from 0 to 30 degrees (Figure 1B). PAI of ≥ 10 mm and ≥ 5 mm were considered excessive in supine and lithotomy positions, respectively.
Overcoming pubic arch interference
In sagittal plane, distances were measured from the perineum to the plane where maximum PAI was found, indicating the required needle insertion depth. If MRI scan length was not sufficient to detect the perineum, the best approximation was made based on the anatomy of patients with sufficient scan length. The maximum amount of PAI defined the upper limit for needle steering, which was compared to the results reported by de Vries et al. [23]. In their study, experiments with a novel steerable needle were performed in tissue simulants and ex vivo bovine tissue. The authors reported lateral steering up to 20 mm over an insertion depth of 100 mm, and similar targeting accuracy for both the steerable needles and conventional rigid HDR BT needles, while adding the ability to steer along curved paths. Figure 2 illustrates the set-up for needle steering in a patient with PAI.
Fig. 2
Prostate brachytherapy set-up with a steerable needle. The steerable needle is steered upwards to access the obstructed volume of the prostate, inaccessible with linear insertion paths
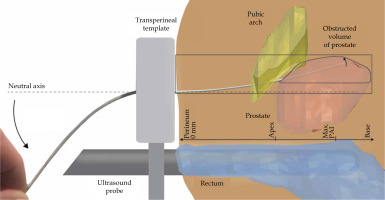
The steerable needle illustrated comprises of a tooled inner needle, having an integrated pull-push mechanism, and a flexible outer needle (ProGuide® sharp 6F needle, Elekta Instrument AB, Stockholm, Sweden). Bending the proximal end of the steerable needle allows for adjustments of the outer needle pathway, and withdrawal of the inner needle creates a work channel for HDR BT.
Results
Pubic arch interference
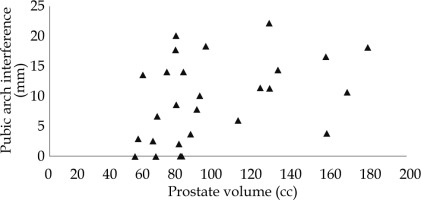
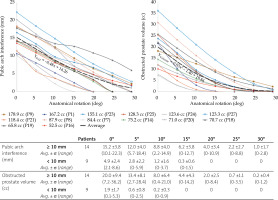
Figure 3 indicates no clear relationship between Vp and PAI. Figure 4 shows the quantification of PAI related to anatomical rotation. In 23 of the 27 patients, the antero-lateral part of the prostate was obstructed, and of these 14 were considered to have excessive PAI in supine position, with PAI (mean ± standard deviation) of 15.2 ±3.8 mm. Four patients had no observed PAI. Change in posture from supine to lithotomy position caused an anatomical rotation of the pelvis that reduced PAI by 4.8 mm after 10 degrees of rotation, and halved PAI with every 15 degrees of rotation. Nevertheless, a 15 degree rotation was still associated with excessive PAI of more than 5 mm in 10 of the 14 cases. These 10 cases had PAI of 8.1 ±2.4 mm. The obstructed volume of the prostate decreased non-linearly with anatomical rotation.
Fig. 4
Quantification of pubic arch interference (PAI) and the obstructed prostate volume related to anatomical rotation. Only patients with PAI ≥ 10 mm in supine position (0o anatomical rotation) based on original MRI scan are shown in the graphs. The dashed lines indicate the average of datasets, which include the corresponding equation. The table describes the amount of PAI and the obstructed prostate volume for all patients with PAI (< 10 mm and ≥ 10 mm)
Needle steering
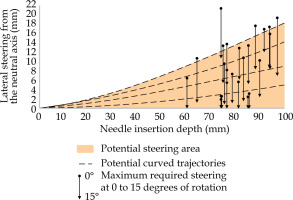
For the patients considered in this study, lateral steering up to 22.3 mm was required over a distance of 73.7 mm in the supine position, and 12.7 mm over a distance of 91 mm after 15 degree rotation. Figure 5 shows that the steerable needle allows for reaching of all the required coordinates after 0 to 15 degrees of anatomical rotation.
Fig. 5
Steerable needle paths to circumvent pubic arch interference (PAI). The amount of interference after 0 and 15 degrees of anatomical rotation is compared to the needle paths from de Vries et al. [23]. The brown area indicates the potential steering area of the steerable needle inside the body of the patient, as illustrated in Figure 2. The dotted black lines show several potential curved needle paths. Steering is applied directly after piercing the perineum, referred to as 0 mm needle insertion depth
Discussion
This work evaluated PAI in patients with large prostates, and demonstrated the effect of anatomical rotation and the potential value of steerable needles to overcome PAI in a simulation study. Our results indicate no clear relation between Vp and PAI, while excessive PAI in the supine position was observed in half of the patients. The change in posture from supine to lithotomy position resulted in improved accessibility of the prostate, but obstructions of more than 5 mm still occurred after 15 degrees of anatomical rotation, which was estimated in literature to relate the supine and lithotomy position [10]. A steerable needle, as presented by de Vries et al. [23], may be able to overcome PAI in these cases allowing for the inclusion of patients with enlarged prostates and excessive PAI in prostate BT protocols.
In some clinical institutions, patients with a prostate of > 50-60 cc are currently considered ineligible for BT. However, large prostates have been successfully implanted with good results [2], while Vp calculations can be inaccurate, which was substantiated by the discrepancy we found between the calculated Vp and the Vp based on the segmented prostate; 4 of the 27 datasets appeared to have a prostate gland of < 60 cc. To avoid exclusion of patients from the BT procedure based on Vp, the GEC-ESTRO ACROP guidelines proposed the evaluation of PAI in enlarged prostates. However, no standard definitions were established for the degree of PAI. Even in small prostate glands, difficulties can arise in combination with a narrow pubic arch [4]. One study noted that 19% of 243 patients with a mean Vp of 44.7 cc were likely to obtain an insufficient dose due to PAI [8]. Peschel et al. reported that 25% of patients required a modified implant [13], and 5.5% of 145 patients in a study of Gibbons et al., having a mean Vp of 46.0 cc, needed ≥ 1 implant needle inserted under an oblique angle without the aid of the needle template, as the pubic arch was obstructing the prostate [14].
The change from supine to lithotomy position can facilitate access to the antero-lateral part of the prostate. This posture change is estimated at 15 degrees, according to Strang et al., based on trigonometry [10], and associated with a decrease in PAI of 4.9 mm, as reported by Tincher et al. [11]. In our analysis, every 10 degrees of rotation caused a reduction in PAI of 4.8 mm. The discrepancy in these findings indicates the variability between patients in either the amount of PAI or in the degree of obtained anatomical rotation from supine to lithotomy position. Either way, it is difficult to pre-operatively predict the amount of PAI occurring during the subsequent procedure. As a general guide, ≥ 10 mm PAI in supine position and ≥ 5 mm PAI in lithotomy position is considered excessive PAI [4, 8, 9]. According to the threshold in supine position, 52% of patients in this work would be considered not directly suitable for prostate BT. When 15 degree anatomical rotation between the supine and lithotomy position was considered, 37% of patients showed excessive PAI.
One solution to make these patients eligible for prostate BT is downsizing the prostate with hormonal therapy, such as ADT. Nonetheless, this therapy takes months, and can result in significant side effects for the patient. Press et al. concluded that hormonal therapy caused worsening of several scores, including quality of life, incontinence, and sexual function, with a tendency to lower vitality [7], whilst Lee et al. stated that ADT may lead to an increased acute urinary morbidity [21]. Other approaches can overcome minimal PAI, such as manipulating rigid implant needles and the ultrasound probe, while extending the lithotomy position can overcome more excessive PAI. Although, patient repositioning requires adaptations to the set-up and can have limitations [16-18].
It should be noted that, in this simulated study, the prostate and the pubic bone rotated equally without the influence of biomechanics; thus, this work indicates the range, to which PAI can occur after posture change from supine to lithotomy position. The maximum PAI and the corresponding insertion depth are taken as a requirement for the end position of the steerable needle, which is compared to the reported curved trajectories. In this experiment, the steering was applied directly after piercing the perineum, while the physician may prefer passing beneath the pubic bone first to diminish the chance of collisions. This would, in fact, reduce the potential lateral steering. In addition, the steerable needle is developed for source positioning in HDR BT. The steering principle can be used also for LDR BT, but requires a re-design to allow for seed implantation. We expect that this would not affect the functionality. The ability to follow the curved trajectories should be evaluated in a clinical setting, as steering was only performed in tissue simulants and ex vivo bovine tissue. In the clinical setting, challenges may arise, including the limited workspace for applying steering and real-time imaging of non-straight implant needles in 3D.
The novel steerable needle as presented in a study of de Vries et al. [23] can overcome PAI, making patients with enlarged prostates and excessive PAI directly suitable for prostate BT without the need for prior hormonal therapy. This spares the patients’ the side effects, and the healthcare system the costs related to hormonal therapy. Additionally, PAI can be evaluated intra-operatively, and ad hoc steering can be used to generate a homogeneous implant geometry in patients with PAI. Further research should be performed in a clinical setting to investigate the dose plans with the use of steerable needles and the corresponding workflow.
Conclusions
The proposed ad hoc steering approach allows for more intra-operative flexibility in needle placement independently from the amount of anatomical rotation and obstruction of the prostate by the pubic arch. This reduces the change of needing to abandon treatment, and suggests that pre-operative hormonal therapy to downsize the prostate may not be necessary. This solution will limit exclusion criteria, and allows patients with enlarged prostates and PAI to consider BT as a treatment option.