Purpose
Among digestive tract tumors, pancreatic head cancer is extremely malignant, with insidious onset and rapid development, and most patients are already in advanced stages when they are diagnosed [1, 2]. Clinically, 40% of pancreatic head cancers are characterized by severe vascularity and extensive local invasion without distant metastasis, which is referred to as locally advanced pancreatic head cancer (LAPHC) [3], making it challenging for radical surgical resection. Even patients who meet the conditions of surgical resection have a high incidence of undetected metastatic lesions, with a rate of 5-year survival of just 25% [4, 5]. The first-line clinical treatment strategy for advanced malignancies is radiotherapy and chemotherapy, but overall survival rate in these patients is still low due to high aggressiveness and heterogeneity of pancreatic cancer, which is particularly prone to drug resistance [6]. Approximately, 30% to 40% of patients die from local progressive destruction of the tumor, not from distant metastases [7]. Majority of patients with pancreatic head cancer often present with symptoms related to progressive aggravated jaundice, and cholestasis often leads to systemic damage, such as severe liver and kidney impairment, immune deficiency, and coagulation dysfunction. Therefore, it is of great importance to relieve biliary obstruction in patients with pancreatic head cancer, who have lost the opportunity for radical surgical resection.
Percutaneous transhepatic cholangial drainage (PTCD) is a common method for interventional treatment of malignant obstructive jaundice, and has been accepted as an effective method for palliative treatment of malignant obstructive jaundice [8]. However, PTCD is limited to palliative treatment of the biliary tract only, and further combined treatment with local implantation of iodine-125 (125I) particles and systemic chemotherapy is necessary. In the past few years, 125I seeds have been implanted minimally invasively into unresectable pancreatic cancer lesions, providing a new and effective treatment option for patients with inoperable pancreatic cancer [9, 10]. 125I damages double-stranded DNA by continuously releasing low doses of ionizing radiation to inhibit its’ proliferation, and eventually lead to cell death. Since the effective radiation radius of 125I particles is 1.7 cm, most of the radiation dose is limited to the interior of the tumor, avoiding damage to surrounding normal tissues [11].
The present study retrospectively analyzed clinical data of 21 inoperable LAPHC patients with obstructive jaundice, aiming to evaluate the clinical efficacy, pain relief, safety, and survival of combined PTCD treatment with 125I implantation and chemotherapy.
Material and methods
Research object
We retrospectively analyzed 21 LAPHC patients with obstructive jaundice admitted to the Department of Nuclear Medicine of the General Hospital of Northern Theater Command from August, 2016 to March, 2021. Inclusion criteria were: 1) Patients confirmed to have LAPHC by abdominal computed tomography (CT) or magnetic resonance imaging (MRI), positron emission tomography (PET)/CT, and other imaging data [12], with LAPHC usually referring to as: a) tumor of the head of the pancreas or the hook; b) tumor invasion around the superior mesenteric artery or the abdominal trunk with a range of > 180°; c) tumor invasion of the first branch of the jejunal artery or invasion/occlusion of the superior mesenteric vein and portal vein, resulting in the inability to reconstruct; d) tumor without distant metastasis; 2) Combined with bile duct obstruction, dilated intra-hepatic bile ducts, and significant gallbladder enlargement; 3) All cases confirmed by cytological or histological biopsy, and not suitable for radical surgery; 4) Complete clinical data; 5) Expected survival > 3 months. Exclusion criteria were: 1) Severe cardio-pulmonary, hepatic, and renal insufficiency; 2) Platelet count less than 50 × 109/l or coagulation dysfunction; 3) Eastern cooperative oncology group (ECOG) physical status score > 2. Except for 2 patients with liver metastasis during the treatment, other patients had no distant metastasis and dual tumors. Hospital’s ethics’ committee approved the study (ethics number: YL2021-07). All patients signed an informed consent form. General patient information are shown in Table 1.
Table 1
Baseline characteristics of patients
Percutaneous transhepatic cholangial drainage
Routine blood analysis, liver, and kidney function, bleeding and clotting time, and electrocardiogram of PTCD patients were examined, and fasted for 4 hours for any obvious contraindications, such as coagulation dysfunction, massive ascites, sepsis, etc. All patients had CT or MR examinations before surgery to evaluate the cause of obstruction, site of obstruction, degree of bile duct dilatation, and the relationship between bile duct and accompanying vessels, and then to determine puncture location and approximate location of the drainage tube. A local low-dose CT scan was performed by applying a localization raster to the skin of corresponding area to precisely mark the skin puncture point and measure the depth of puncture and the angle of needle entry, paying attention to avoid large blood vessels. After routine disinfection and local anesthesia, subcutaneous tissue was punctured with an 18 G puncture needle to the upper edge of the rib cage, and the patient was instructed to quickly puncture the hepatic peritoneum along the predetermined direction and angle during CT localization while closing the air. If there was no bile outflow, the bevel direction of the needle tip, the depth, and the angle of needle entry would be slightly adjusted locally and then check for a bile outflow. After confirming that the needle tip was located in the bile duct, a 0.035 inch super-slip guidewire was inserted and placed as far as possible into the common bile duct or significantly dilated hepatic duct to drain as much of larger area as possible. The dilated duct was introduced along the guidewire and repeatedly flushed with antibiotics; if the right and left hepatobiliary ducts were not connected, the other side was punctured. An 8 F drainage catheter was introduced along the guidewire, and the apex of pigtail drainage tube was locked to hold the drainage tube in place.
125I particle implantation
Patients underwent PTCD for 1 week to review the liver and kidney function, electrolytes, routine blood analysis, coagulation four and other routine examinations. After the liver function was recovered significantly, an enhanced CT scan was performed to prepare patients for the implantation. An enhanced CT of the abdomen (within 1 week) was introduced into treatment planning system (TPS) to accurately outline gross target volume (GTV) and organs at risk (OARs), including duodenum, stomach, spinal cord, bilateral kidneys and other vital organs. These were confirmed by the imaging physician, physicist, and nuclear medicine physician, and range of clinical target volume (CTV) was designed according to GTV and surrounding normal sensitive organs. Planning target volume (PTV) was 1 cm outside CTV, the edge of PTV was covered by 90% isodose line, and the prescribed dose was 90-120 Gy. Implanted particles were uniformly distributed in the lesion according to TPS plan. After completion of the procedure, the puncture site was pressed for 10-20 minutes, and CT scan was performed again to exclude bleeding and other complications. Post-operative fasting in bed for 24 hours, hemostatic drugs, and growth inhibitors were routinely applied to avoid complications, such as bleeding and pancreatic fistula.
Radioactive particles were purchased from Chengdu Yunke Pharmaceutical Co., Ltd. (Chengdu, China). Iodine-125 seed source is a sealed source of radionuclides. The parameters of 125I seeds were as follows: activity of 0.4~0.7 mci, the diameter: 0.8 mm, half-life: 59.6 days, primary radiation: 31.4/27.4 keV X-ray and 35.5 keV γ-ray; the seeded surface was covered with titanium alloy. Particle implantation scanning machine used was a 64-row CT conventional scanning configured by Discovery VCT PET/CT (GE Company, USA). TPS was provided by Beijing Feitian Zhaoye Technology Development Co., Ltd. (China), and the median number of implanted particles calculated from TPS was 48.5 (range, 15-112).
Chemotherapy
Gemcitabine hydrochloride for injection (1,000 mg/m2) was administered intravenously on days 1 and 8 of chemotherapy, and repeated every 3 weeks. Capecitabine tablets (1,000 mg/m2• d) were administered orally on days 1~14 of chemotherapy, and repeated every 3 weeks, with continuous treatment for 3~4 weeks.
Follow-up visits
Two-three days after surgery, CT was reviewed to show the location of 125I particles and local complications. Routine follow-up hematology and imaging were performed every 2 months after PTCD, 125I particle implantation, and chemotherapy combination. Follow-up parameters included hematological examinations (white blood cell [WBC], platelet [PLT], serum total bilirubin [TBIL], serum direct bilirubin [DBIL], serum alanine aminotransferase [ALT], serum aspartate aminotransferase [AST], serum alkaline phosphatase [AKP], albumin [ALB], carcinoembryonic antigen [CEA], cancer antigen 199 [CA-199], cancer antigen 125 [CA-125]), clinical manifestations (related to biliary obstruction and symptoms as well as signs associated with pancreatic head cancer) and imaging (MR or CT). Survival was assessed based on time of death or follow-up cut-off time. Follow-up was achieved through return visits in clinic or by telephone and online contacts to imaging and hematology results as well as laboratory tests performed at the hospital.
Statistical analysis
All data were analyzed using SPSS v. 20.0, and normally distributed measures were expressed as mean ± standard deviation (x ± s) using t-test or Wilcoxon test. Count data were expressed as rate (%) using χ2 test or Wilcoxon test. The difference of p < 0.05 was considered statistically significant.
Results
Evaluation of efficacy
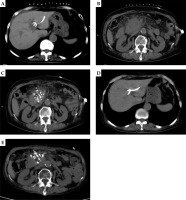
All 21 patients had biliary drainage, and received a total of 59 cycles of chemotherapy sessions of gemcitabine (GEM) combined with capecitabine tablets, with an average of 2.1 cycles per patient. The CT review 2 months after the combination chemotherapy treatment showed 3 cases of complete response (CR), 12 cases of partial response (PR), 3 cases of stable disease (SD), and 3 cases of progressive disease (PD), with an overall efficiency of 71.43%, and a local control rate of 85.71% (Fig. 1). The median post-operative particle implantation was 54.2 (range, 18-125), with no statistically significant difference compared with the pre-operative TPS plan (p > 0.05).
Fig. 1
A) A 73 years old female. Computed tomography (CT) scan shows CT-guided percutaneous transhepatic cholangial drainage (PTCD) in pancreatic head cancer complicated with obstructive jaundice. B) CT scan shows the maximum diameter of pancreatic head cancer of about 75.1 × 52.5 mm. C) CT scan shows CT-guided percutaneous radioactive seed implantation for pancreatic head carcinoma. D) CT scan shows the bile duct dilatation significantly improved after PTCD drainage. E) CT scan shows the lesions of pancreatic head cancer rechecked after 2 months of treatment, and the maximum diameter was about 47.7 × 38.9 mm. Evaluate partial response (PR)
Clinical benefit: The assessment of clinical benefit efficacy, which included improvement in clinical symptoms and the occurrence of complications
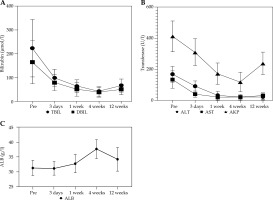
Jaundice, abdominal pain, and abdominal distension of the 21 patients improved to different degrees after surgery, and the skin pruritus disappeared. Bilirubin and transaminase levels improved to varying degrees at 3 days, 1 week, and 4 weeks after treatment, and the difference was statistically significant compared with that before treatment (p < 0.05). Although the value of liver function at 12 weeks after treatment increased compared with that at 4 weeks after treatment, it was still statistically significant compared with the value before treatment (t = 10.928, 9.237, 12.523, 11.924, 6.256, p < 0.05). ALB level decreased slightly 3 days after treatment, and improved 4 weeks after treatment, with no significant difference (p > 0.05) (Table 2, Fig. 2).
Table 2
Changes in laboratory values after stenting (x ± s)
Pain improvement
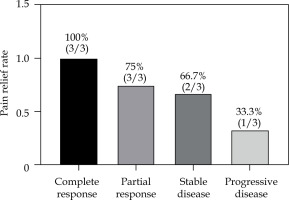
The pain relief was statistically significant one month after treatment compared with that before treatment (VAS scores: 6.76 ±2.25 vs. 3.25 ±1.92, p < 0.001), and the rate of pain relief was 71.43% (15/21). In addition, of the 3 patients with severe pain before treatment, one was downgraded to moderate pain, one to mild pain, and one patient had no change. Of the 11 patients with moderate pain before treatment, complete relief of pain was observed in two patients, downgraded to mild pain in seven, and unchanged in two. Of the seven patients with mild pain before treatment, four had complete pain relief, and the remaining three had no change after implantation (Table 3, Fig. 3).
Complications
There were no perioperative deaths and no severe complications as follows in 21 patients: acute and chronic pancreatitis, gastrointestinal bleeding, biliary fistula, abdominal infection, pancreatic fistula, or acute failure of liver and kidney function after surgery. In four patients, intra-operative bleeding occurred, two had a small amount of bleeding from the drainage tube on the first post-operative day, 5 patients experienced gastrointestinal symptoms, such as abdominal distension, abdominal pain, and nausea, and five patients had fever. All the patients improved after symptomatic treatment.
Comparison of serum CEA, CA-199, and CA-125 levels before and after treatment
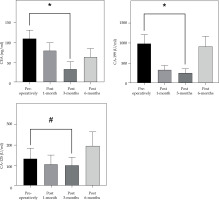
The mean serum CEA, CA-199, and CA-125 levels were determined to be significantly lower in 21 patients at 3 months post-treatment compared to pre-treatment, with statistically significant differences (p < 0.05, Fig. 4).
Fig. 4
Comparison of carcinoembryonic antigen (CEA), cancer antigen 199 (CA-199), and cancer antigen 125 (CA-125) levels between pre- and post-treatment (x ± s). The abscissa represents the patients after treatment, and the ordinate represents the tumor marker level. The CEA level was 109.15 ±22.36 ng/ml pre-operative and 33.46 ±17.98 ng/ml at 3 months post-treatment. CA-199 level was 988.82 ±226.53 U/ml pre-operative and 254.56 ±98.22 U/ml at 3 months post-treatment. CA-125 level was 132.63 ±52.09 ng/ml pre-operative and 100.52 ±39.88 ng/ml at 3 months post-treatment
*Significant difference in CEA/CA-199 level between pre-therapy and post-treatment (t = 10.378, 9.525, p < 0.01), #difference in CA-125 level between pre-therapy and post-treatment (t = 3.262, p < 0.05)
Survival period
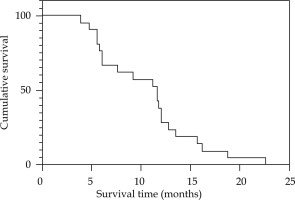
The median duration of follow-up was 12 months (range, 4-25 months). Two patients had recurrent obstructive jaundice due to intra-hepatic metastases forming hilar obstruction, who were re-treated with PTCD to reduce the jaundice. The 6-month and 12-month survival rates were 66.7% and 28.6%, respectively. By March, 2022, all 21 patients had died. The causes of death included multiple organ failure, infection, distant metastasis, etc. The overall survival time was 3.9-22.6 months, and the median survival time was 11.6 months (95% CI: 4.08-24.52%) (Fig. 5).
Discussion
Most patients with pancreatic head cancer lose the opportunity for surgical radical treatment at the first visit due to symptoms of obstructive jaundice caused by insidious onset of the disease [13]. Due to the deep location of the pancreatic structures, the dose of conventional external irradiation is limited; therefore, local control is poor and it is difficult to achieve a better outcome [14]. Patient’s liver and kidney function and coagulation are significantly impaired, so systemic chemotherapy is also difficult to perform. At this point, it is necessary to first relieve obstructive jaundice and then treat the pancreatic head cancer lesion. The most effective method of palliative treatment for malignant obstructive jaundice is currently recognized as PTCD [8, 15], which can reduce the serum bilirubin level within a short period of time, and successful implementation of this technique allows for bile to be drained out of the body or to return to the intestine. With an adequate discharge of stagnant bile, the effect of damage to liver cells is gradually reduced, liver function is improved, and the accompanying pruritus and other uncomfortable symptoms are relieved. In this study, PTCD was performed in all 21 patients of the study group, showing a 81.17% decrease in TBIL, 76.63% decrease in DBIL, 88.09% decrease in ALT, 84.00% decrease in AST, 71.52% decrease in AKP, and 20.21% increase in ALB 1 month after PTCD. However, PTCD has no therapeutic effect on the tumor itself and malignant obstructive jaundice. As the primary lesion of pancreatic head cancer continues to grow, the tumor compresses the drainage tube or even blocks the lateral hole of the drainage tube, which easily cause obstructive jaundice again, and at this time, effective treatment methods should be actively adopted to treat the primary lesion of pancreatic head cancer [16].
Radioactive 125I particle implantation therapy has been widely used clinically in various solid malignant tumors for many years, with the characteristics of targeted treatment of tumors, and has achieved positive clinical efficacy. Experts’ consensus on radioactive 125I seeds interstitial implantation brachytherapy for pancreatic cancer [17] argued that the clinically recommended tumor matched peripheral dose (MPD) should be between 80 Gy and 145 Gy. Since pancreatic tumors are located in the retroperitoneum and the surrounding normal tissues, stomach, and duodenum are the organs at risk that may be endangered by particle implantation, ensuring the safety of the surrounding normal tissues is a prerequisite for giving pancreatic tumor particle implantation. In our case, the tissues and organs surrounding the lesion showed great respiratory and cardiac activity. In addition, high prescription doses affect tumors surrounded by complex blood vessels (blood vessels, lymphatics, bile ducts, and pancreatic ducts), and low prescription doses prolong the course of treatment and are not conducive to shrinking the mass as soon as possible, providing rapid relief of pain and jaundice. Therefore, we chose to prescribe a dose of 90-120 Gy. In this study, 21 patients with pancreatic head cancer implanted with 125I particles under PET/CT guidance combined with chemotherapy also achieved good local efficacy, and the assessment after 2 months of combined treatment showed an overall efficiency of 71.43% (15/21) and a local control rate of 85.71% (18/21). The pain relief was statistically significant one month after treatment compared with that before treatment (VAS scores: 6.76 ±2.25 vs. 3.25 ±1.92, p < 0.001), and the rate of pain relief was 71.43% (15/21). Huang et al. [18] proposed a pain relief rate of 80.6% (58/72) with particle combined with chemotherapy for advanced pancreatic cancer, and suggested a median progression-free survival (mPFS) of 9.5 months and median overall survival (mOS) of 12.5 months. Li et al. [19] investigated 50 patients with incurable pancreatic cancer subjected to intra-operative ultrasound-guided particle implantation, 26 of whom received GEM + albumin after particle and GEM + albumin paclitaxel chemotherapy again, with mOS of 14 and 11 months for patients who received and did not receive chemotherapy, respectively (p = 0.046). Chi et al. [20] selected 21 patients with pancreatic head cancer accompanied by obstructive jaundice, all of whom underwent biliary stenting with 125I particles combined with chemotherapy. The overall survival of these 21 patients was 5.2-23.3 months, which was similar to that of the present study. Chen et al. [21] selected 42 LAPHC patients with obstructive jaundice, and twenty-two patients underwent CT-guided 125I seed placement after biliary stenting via percutaneous transhepatic biliary drainage (PTBD). The overall positive response rate at 6 months after treatment was 72.7% (16/22) and 30% (6/20) in patients who received and did not receive particle implantation, respectively. This efficacy may be related to the following characteristics of 125I particles: the effective distance of radioactive 125I particle radiation is short, and most of the radiation energy is absorbed by the tissues inside the lesion; the radiation source is located inside the tumor, and γ-rays released from the particles destroy the tumor cells through direct and indirect effects, thus killing the tumor cells; because of direct irradiation, the damage to normal tissues is greatly reduced, and the tumor cells are killed to the maximum extent. Moreover, compared with external radiotherapy, low-dose-rate brachytherapy has the following advantages: the repair of sublethal damage during radiotherapy can relatively protect normal tissues; tumor cells would not regenerate; cell cycle can be re-distributed; low oxygenation rate. In addition, CA-199 is the only marker currently approved by the US FDA for pancreatic cancer, and is the most used indicator to validate patients with symptomatic pancreatic cancer [22]. Tsai et al. [23] found that reduction of CA-199 is a valid marker of successful neoadjuvant therapy for pancreatic cancer, and its’ normalization is the strongest prognostic marker for long-term survival. In this paper, 21 patients showed varying degrees of reduction in CA-199, CEA, and CA-125 after combination therapy, with statistically significant differences (t = 9.525, 10.378, 3.262, p < 0.05). No serious complications, including acute and chronic pancreatitis, gastrointestinal bleeding, biliary fistula, abdominal infection, pancreatic fistula, or acute failure of liver and kidney function, occurred after surgery, reflecting the operational safety and ease of control of complications of this study method. One patient showed gastrointestinal reactions, such as nausea, vomiting, poor appetite, and abdominal distension for a longer period of time after surgery, which was considered to be radiological inflammation that improved after treatment with gastrointestinal mucosal protective agents and other drugs.
In conclusion, all patients in this study completed the radioactive particle implantation surgery according to the pre-operative treatment plan, and there was no perioperative death in the whole group, which minimized the tumor load and eliminated the clinical symptoms caused by metastatic lesions under the premise of safe and minimally invasive treatment. The application of PTCD combined with particle implantation and chemotherapy for the treatment of LAPHC patients with obstructive jaundice achieved better results. The average survival of the 21 patients in this study was 11.6 months after treatment, indicating that combination therapy for LAPHC patients with obstructive jaundice is clinically effective and improves the quality of life of patients.