Introduction
Reflectance confocal microscopy (RCM) involves the formation of reflected light under laser excitation. It can perform tomographic scanning on cells or tissues and the signal can be imaged using a computer. The technique allows the observation of three-dimensional sections of the skin at will; hence, it is also known as skin three-dimensional computed tomography (CT) [1]. The images obtained have different brightness and darkness according to different skin structures such as melanin and organelles. Furthermore, the melanin and keratin in the skin have higher refractive indexes. Thus, the technique allows non-invasive, real-time, and dynamic scanning imaging of the skin structures [2–5]. Further, because the technique allows repeated examination of the same skin area without causing any damage, it can be used to monitor progression and treatment outcomes for diseases such as vitiligo and psoriasis [6, 7]. RCM allows imaging of a cross-section of the skin, with each layer being 1–5 μm in thickness and horizontal resolution of 0.5–1.0 μm. The maximum depth of imaging afforded by RCM is approximately 350 μm, and the imaging range could extend from the skin surface to the dermal papilla layer [8]. RCM images are easy to collect and preserve, which is convenient for comparing lesion development during a long-term follow-up [9, 10]. Therefore, RCM is considered to be the most promising imaging tool for the evaluation of skin lesions. Many diseases have characteristic RCM images, especially pigmented diseases [11–13], inflammatory diseases [14, 15], and skin tumours [16–18].
Application in dermatology
Pigmented skin disease
Hypopigmentation disease
Vitiligo is a common congenital or generalized skin pigmentation disease. Its skin lesions are milky white, with a smooth surface without rash, and the white spots are clear. The RCM features of vitiligo are as follows: complete or partial loss of pigmentation in the lesion area, loss of the basal layer pigment ring, and slight inflammatory cell infiltration in the superficial dermis [19, 20]. In the advanced stages of vitiligo and in the early stage of clinically atypical lesions, the use of RCM to detect the loss or partial deletion of the pigment ring is of great significance for the early diagnosis of vitiligo. The efficacy of vitiligo treatment can be evaluated by RCM based on the regeneration of the pigment from around the hair follicle and the active proliferation of melanocytes around the lesion. In the conditions considered during the differential diagnosis of vitiligo, such as achromic nevus, and nevus anemicus: hypopigmentation or approximately normal, the basal pigment ring is intact can be observed using RCM [21, 22].
Senile leukoderma is common in middle-aged and elderly people. Compared to vitiligo, it is characterized by the following findings on RCM: loss of the pigment ring structure of the surrounding normal skin. Pityriasis alba is more common in children and characterized by round or oval white spots with a clear boundary. The main features of pityriasis alba on RCM are as follows: hypopigmentation in the lesion area but not completely missing. In cases of postinflammatory hypopigmentation, RCM revealed melanophages; this finding is not consistent with the RCM findings of vitiligo. In addition, the content of melanin and pigment ring depends on the depth of inflammation [6].
Hyperpigmentation diseases
Chloasma occurs in young and middle-aged women, and the skin lesions often appear as yellow or dark-brown patches on the face, and are often symmetrically distributed on the cheeks. The histopathological features of chloasma are as follows: active melanogenesis in the epithelial basal layer and presence of free melanin particles in the upper part of the dermis. The features of chloasma on RCM are as follows: the pigment content in the basal layer of the epidermis significantly increased in the lesion area and some pigment granules were deposited in the superficial dermis of some patients. Nevus fusco-caeruleus zygomaticus (Figure 1 A) and nevus of Ota (Figure 1 D) manifest as brown or dark-grey pigmentation on the face. RCM findings of the conditions are as follows: normal pigmentation in the epidermal layer and dermis layer characterized by the distribution of strip-like highly refractive coloured pigment agglomerates. Cord-like or dendritic melanocytes are visible between the dermal and middle collagen fibres, and there is no inflammatory cell infiltration (Figures 1 B, E). The histopathological features of the conditions are as follows: melanocytes in the basal layer of the epidermis are larger, and there are increased numbers of melanin particles in the basal cells (Figures 1 C, F). Riehl melanosis is a type of photocontact dermatitis that manifests as brown or blue-grey flaky pigmentation on the face. Its histopathologic features mainly consist of liquefaction and degeneration of the basal layer, dermal perivascular inflammatory cell infiltration, and presence of large numbers of melanin particles within and outside melanocytes. The RCM findings of the condition are as follows: basal cell liquefaction and degeneration, increased number of mononuclear cells in the dermal papilla, and phagocytic cell infiltration [23, 24].
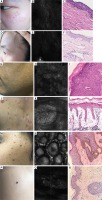
Figure 1
Representative photos of several common skin diseases. A – Naevus fusco-caeruleus zygomaticus: an 18-year-old girl with brown spots on the face. B – RCM features of naevus fuscocaeruleus zygomaticus: cord-like high-refractive pigment masses in the dermis, and cord-like or dendritic melanocytes between collagen fibre bundles in the dermis. C – Histopathologic features of naevus fusco-caeruleus zygomaticus: a few scattered spindle-shaped melanocytes between collagen bundles in the superficial reticular dermis. D – Naevus of Ota: a 23-year-old female with facial black patches. E – RCM features of naevus of Ota: cord-like high-refractive pigment masses scattered in the dermis. F – Histopathologic features of naevus of Ota: a large number of spindleshaped, dendritic, or stellate melanocytes gathered between the collagen bundles in the dermis, sometimes extending into the papillary layer or subcutaneous tissue G – Verruca plana: a 30-year-old man with multiple skin-coloured papules on his face. H – RCM features of verruca plana: the upper parts of the squamous layer of the spinous layer has a concentric annular structure. I – Histopathologic features of verruca plana: obvious hyperkeratosis and acanthosis, vacuolization of the upper epidermis cells, and stratum corneum cells showing obvious mesh-like appearance due to vacuolization. J – Psoriasis: a 38-year-old man has multiple red papules on his back and a small number of scales on the papules. K – RCM features of psoriasis: hyperkeratosis, parakeratosis, thickening of the spinous layer, capillary dilatation and hyperaemia, and peripheral inflammatory cell infiltration. L – Histopathologic features of psoriasis: hyperkeratosis and acanthosis, neutrophils agglomeration. M – Seborrheic keratosis: a 56-year-old woman has multiple brown papules on her face. N – RCM features of seborrheic keratosis: epidermal brain-like structure, bright cobblestone-like structure of the stratum corneum, superficial vasodilatation, and hyperaemia of the dermis. O – Histopathologic features of seborrheic keratosis: hyperkeratosis, acanthosis and papillary hyperplasia. P – Nevomelanocytic nevi: a 30-year-old man with a brown macule on the shoulder. Q – RCM features of nevomelanocytic nevi: a normal epidermal structure, bright cobblestonelike structure in the basal layer, and a circular or elliptical nested arrangement. R – Histopathologic features of nevomelanocytic nevi: nevus cells nesting in the dermis
Inflammatory skin disease
Inflammatory skin diseases include both infectious and non-infectious skin diseases.
Infectious skin diseases such as verruca plana often develop on the face, as flat or bulging pale brown papules (Figure 1 G). The general RCM findings for infectious skin diseases are as follows: the upper layers of the squamous layer of the spinous layer are concentric annular structures [14] (Figure 1 H). The histopathological features of the conditions are as follows: Hyperkeratosis, acanthosis, vacuolar cells (Figure 1 I). Molluscum contagiosum is more common in children, and is characterized by the development of pearly hemispherical papules 3–5 mm in size and central umbilical concavity. The RCM findings are as follows: complete cystic cavity-like structures in the epidermis and bright circular cells in the lumen. The RCM results for varicella and herpes zoster include the following: blister formation in the epidermis, and highly refractive disc-shaped cells in the blebs [25]. In cases of superficial fungal infections, hyphae and spores can be seen under RCM [26, 27].
Non-infectious skin diseases such as psoriasis are common and are chronic, recurrent inflammatory skin diseases. The lesions of psoriasis can occur anywhere in the body, usually symmetrically distributed [28] (Figure 1 J). The characteristics under RCM include hyperkeratosis, parakeratosis, thickening of the spinous layer, accumulation of cells with a lobular nucleus and expansion of the dermal papilla in dilated vessels [29, 30] (Figure 1 K). The histopathological features of psoriasis are as follows: hyperkeratosis, parakeratosis, thickening of the spinous layer, capillary dilatation and hyperaemia, and peripheral inflammatory cell infiltration [31, 32] (Figure 1 L).
Pityriasis rosea is an acute inflammation skin disease and skin lesions with scaly rose-coloured rash [33, 34]. Its histopathological features include superficial perivascular inflammatory cell infiltration of the dermis, epidermal keratosis, and thickening of the basal layer [35]. The RCM findings are as follows: thickening of the spinous layer, localized blister formation, and slight inflammatory cell infiltration.
Lichen planus lesions are polygonal and flat characterized by purple-red papules with clear boundaries [36]. The main pathological feature is basal cell liquefaction and denaturation [37]. RCM findings of lichen planus include a thickened granular layer, acanthosis, liquefaction and degeneration of the basal layer, and infiltration of dermal papillary phagocytes and inflammatory cells [38].
Dermatitis and eczema are marked by recurrent chronic skin inflammation. These conditions are mainly characterized by erythema and papules. The histopathological features include hyperkeratosis, parakeratosis, superficial capillary expansion of the dermis, and infiltration of inflammatory cells around blood vessels [39]. The RCM findings are as follows: epidermal cell and intracellular oedema, oedema formation in the spinous layer and under the horn, and dermal papillary vasodilation.
Discoid lupus erythematosus is an autoimmune disease. The lesions are marked by clear erythema, with the main histopathological feature being basal cell liquefaction and denaturation [40]. The RCM findings are as follows: hyperkeratosis, acanthosis, inflammatory cell infiltration, basal cell liquefaction and denaturation [41].
Skin tumours
Seborrheic keratosis is benign hyperplasia of the epidermis due to the proliferation of keratinocytes. It occasionally develops on the face and is mostly characterized by pale brown rash or flat papules, the number of which increases with age and seriously affects the appearance of the affected individual (Figure 1 M). The RCM findings include epidermal brain-like structures, bright cobblestone-like structure of the stratum corneum, superficial vasodilatation, and hyperaemia of the dermis [42, 43] (Figure 1 N). The histopathological features of seborrheic keratosis are as follows: hyperkeratosis, acanthosis, papillary hyperplasia, and hyperplastic tumour tissue consisting of squamous cells and basal-like cells (Figure 1 O).
Nevomelanocytic nevi is a benign lesion consisting of nevus cells. It is a common skin disorder with many types of clinical manifestations. The condition is characterized by lesions that are mostly dark brown or black in colour and a few colourless spots (Figure 1 P). RCM findings include a normal epidermal structure, bright cobblestone-like structure in the basal layer, and circular or elliptical nested arrangement [44], which can be classified according to the level of the sputum cells [45] (Figure 1 Q). The histopathological features of nevomelanocytic nevi are as follows: nevi cells at different levels of the skin (Figure 1 R).
Porokeratosis is a rare, hereditary chronic progressive keratoderma dermatosis. The main features of the skin lesions are edge protrusion and mild central atrophy. The histopathological features of porokeratosis are as follows: intraepithelial keratinization of the cell column; reduction or disappearance of the granular layer under the horn plate layer; and chronic inflammatory cell infiltration along dilated perivascular vessels in the dermal capillaries. The RCM findings are as follows: peripheral low refractive bulge, disordered epidermal structure, and infiltration of inflammatory cells in the superficial dermis [46].
Basal cell carcinoma (BCC) is one of the most common types of skin cancer. It is an epithelial low-grade malignant tumour derived from epidermal basal cells or the outer root sheath of hair follicles. It most commonly develops in the elderly. The carcinoma initially develops as a small nodule, which eventually spreads and appears dark brown; the lesion centre eventually ruptures, presenting an invasive ulcer with a curled pearly edge and a black base. Early diagnosis and treatment can reduce the risk of metastasis and improve prognosis. The histopathological features of BCC include cancer cells like basal cells, cancer cells in the dermis connected to the epidermis, connective tissue proliferation around the cancer cell mass, and mucin degeneration. The RCM findings are as follows: obvious inflammatory cell infiltration, vascular distortion, parakeratosis, intercellular oedema, bright dendritic cell structure in the basal layer [47, 48].
Squamous cell carcinoma (SCC) is the second most common skin malignancy. SCC originates from the epidermis of the skin and keratinocytes; it is characterized by early ulceration, often secondary to chronic ulcers. The opening of the sinus or the ulcer becomes cancerous if it does not heal over a prolonged period. There may be local infiltration and regional lymph node metastasis. Therefore, early diagnosis and treatment are essential [49]. The histopathological features include irregular tumour cells constituting a cancer nest invading the dermis. The tumour cell mass is composed of different proportions of atypical squamous cells and normal squamous cells. The RCM findings are as follows: disappearance of the local honeycomb cell structure, circular nucleated cells in the superficial epidermis, increase in the dendritic structure, and dermal papillary vasodilation [50].
Malignant melanoma (MM) is the most malignant type of cancer; it is highly prone to metastasis and is the leading cause of mortality among skin tumours. Its histopathological features include an increased scattering of melanoma cells in the epidermis and dermis. The use of RCM can improve the rate of diagnosis of MM. Furthermore, the use of the technique is acceptable to patients because of its advantageous characteristics such as no damage, no pain, and rapid diagnosis. RCM usually indicates the disappearance of the honeycomb cell structure. Additionally, bright layers of bright cells are seen in the layers of the epidermis as well as highly luminous melanocytes in the basal layer, disappearance of the pigment ring, increased mononuclear cell infiltration, and blood vessel expansion and congestion in the dermal papilla layer [51, 52] (Table 1).
Table 1
A brief history of RCM
Advantages of RCM
Clinically, most patients refuse to undergo pathological examinations because they can be traumatic, expensive, and time-consuming. Most patients choose non-invasive examination that can provide results instantly. Compared with pathological examinations, RCM has more advantages: it is non-invasive, real-time, dynamic, easy to conduct, reproducible, and has a short operation time [53, 54] (Table 2). However, it cannot replace histopathological examinations, which are the gold standard for disease diagnosis.
Conclusion and perspectives
RCM is a modern imaging technique that enables the non-invasive characterization of skin lesions located in the epidermis and superficial dermis with high resolution. It is easy to perform, painless, can be used for a wide range of indications, and provides results in a timely manner. RCM is considered to be the most promising imaging technique for the quasi-microscopic morphological and dynamic characterization of superficial skin lesions. Furthermore, the images are easy to collect and preserve, which is convenient for comparing the development of lesions during long-term follow-up and helpful for a multi-dimensional and stereoscopic understanding of the traits of lesions and for dermatological diagnoses. The technique allows sequential non-invasive examination of the same skin area without causing any damage and monitoring of disease progression and treatment outcomes. Three-dimensional CT of the skin has shown good application prospects, especially in the diagnosis of pigmentation disorder and inflammatory diseases. If RCM is used as a routine examination method, the diagnostic accuracy of skin diseases can be improved. With the clinical application and promotion of RCM, it is believed that clinical diagnosis of skin lesions can achieve satisfactory results.








