Purpose
Uveal melanoma is the most frequent primary malignant intraocular tumor among adult population [1]. Although this tumor can be found in all parts of the uveal tract, iris location is the least common, with an incidence of 3-5% of all reported cases [2-4]. Iris lesions exhibit slow growth over time so to differentiate between nevus and melanoma can be challenging in some cases. Due to the low metastatic potential [5, 6], observation of suspicious naevi for documented growth is considered safe and standard procedure in most ocular oncology centers. Some years back, an ABCDEF guide was introduced to evaluate suspected iris lesions (A – age under 40 years, B – blood in anterior chamber, C – clock hour inferior, D – diffuse configuration, E – ectropion uveae, F – feathery margin) [7] and to help to determine their character. Moreover, gonioscopy and ultra-biomicroscopy (UBM) with assessment of iridocorneal angle (ICA) are inclu-ded in the assessment of melanocytic iris lesions. ICA involvement significantly increases the risk of metastasis at 10 years [8].
Recent studies have added some insight into the iris melanoma genetic background. Van Poppelen et al. found that iris melanomas are a distinct sub-group of melanocytic lesions, harboring genetic mutations comparable to not only choroidal, but also cutaneous melanomas. These mutations include changes in genes: GNAQ, GNA11, EIF1AX, SF3B1, and BAP1. What is interesting, unlike in choroidal melanoma, the presence of BAP1 mutation does not influence the prognosis in the iris melanoma group [9].
Ruthenium-106 (106Ru) plaque brachytherapy is a method of conservative therapy for iridociliary melanoma among other treatment options, such as local excision (iridocyclectomy), iodine-125 (125I), palladium-103 (103Pd) plaque brachytherapy, and proton beam radiation therapy (PBRT) [5, 6, 10-12]. Irradiation of iris tumor is considered a treatment of choice for lesions involving an angle with more than 2-clock hours, therefore non-resectable.
The aim of this study was to review the single-center long-term outcomes of 106Ru brachytherapy for iris and iridociliary melanoma.
Material And Methods
Medical records of patients who received 106Ru plaque treatment for iridociliary melanoma at the Department of Ophthalmology, Poznań University of Medical Sciences between 1999 and 2021, were retrospectively reviewed. The diagnosis of iris melanoma in 20 patients (83%) was made after documented growth of the observed melanocytic lesion, and 3 patients (12%) presented with clinically evident signs of malignancy (hyphema, unilateral glaucoma). In one patient (4%), a biopsy of the lesion was performed due to atypical clinical presentation of the tumor confirming a spindle cell melanoma. Exclusion criteria were diagnosis of other malignancy confined to the iris (i.e., metastasis), tumor size exceeding CCB 106Ru plaque diameter and patient inability to undergo a surgical procedure.
Surgical technique
Dose calculation was estimated with the depth of anterior chamber to deliver 80-100 Gy to the tumor base. If the tumor was extending to the angle, it was covered with a 20 mm or 15 mm 106Ru plaque (Eckert & Ziegler BE-BIG GmbH, Berlin, Germany) with a 2 mm margin. Post-ope-ratively, a contact lens was placed over the plaque. For tumors infiltrating the entire iridocorneal angle, the plaque was placed centrally on the cornea to irradiate the entire anterior segment, and then covered with conjunctival Gundersen flap. After plaque removal, all patients were observed following the same routine, starting on the seventh post-surgical day. Cataract removal, if necessary, was a standard phacoemulsification procedure, with an in-bag single-piece acrylic lens implantation.
Results
We identified 24 patients with iris and iridociliary melanoma. Their demographic features are listed in Table 1. The median time of observation prior to treatment was 3 months (range, 0-68 months). Twenty-three tumors (96%) were melanocytic and one (4%) was amelanotic. The median height of the tumor was 1.85 mm (range, 0.4-3.0 mm). Eight tumors (33%) extended to the iridocorneal angle. Based on the AJCC 8th edition classification for iris melanoma, 15 tumors (62%) were graded T1a, one (4%) was T1b, 6 (25%) were T2a, and 2 tumors (8%) were graded T2c.
Table 1
Demographic features (N = 24)
After melanoma diagnosis, 19 (79%) patients received treatment with 20 mm CCB plaque, and 5 patients (21%) were treated with 15 mm CCA plaque. One patient (4%) received primary total irradiation to the iridocorneal angle due to an extensive iridocorneal angle invasion. The median sclerocorneal dose (to the base) was 238 Gy (range, 165-348 Gy), the median dose to the base of the iris tumor (apical dose) was 94 Gy (range, 83-108 Gy). Table 2 presents the tumor height, doses received by tumor and sclera, and time of irradiation for 22 patients. Two patients lacked complete dosimetric data due to an archival data loss in the past, and were not included in the table. Figures 1-3 show the follow-up pictures of the patients before and/or after irradiation with 106Ru plaque.
Table 2
Dosimetric data for the tumor apex and adjacent scleral tissue
Fig. 1
63-year-old woman, 106Ru brachytherapy in 1998. A) Slit-lamp photography of pigmented lesion in 2008; B) 2014; C) 2018; D) 2022. Best corrected visual acuity (BCVA) in 2023 – HM (declined cataract surgery). Note: Stable keratopathy
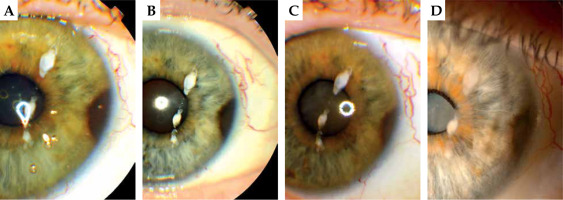
Fig. 2
37-year-old woman, first visit and follow-up after 106Ru irradiation in 2019. A) 2019, slit-lamp photography of a pigmented lesion of the iris; B) The same visit, ultra-biomicroscopy (UBM); C, D) 2021, after irradiation, the same visit; E) 2022, tumor regression
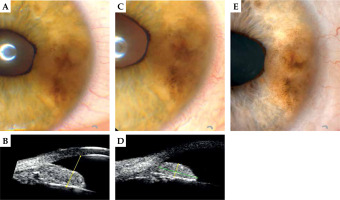
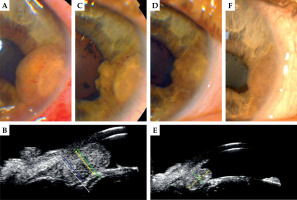
Fig. 3
46-year-old man, 106Ru brachytherapy in 2019. A) Slit-lamp photography of the iris melanoma before treatment; B) ultra-biomicroscopy (UBM) before treatment; C) The same year, 6 months after irradiation, tumor regression; D, E) 2021; F) 2022. Best corrected visual acuity (BCVA) in 2023 – 0.4
The median follow-up time was 67.5 months (range, 24-265 months). We noted one (4%) recurrence of a diffuse iris melanoma 2 years post-primary treatment that was managed with an subsequent irradiation of the whole anterior segment. Five years after the second procedure and cataract removal, the eye is salvaged with no sign of local recurrence and maintains the vision of 0.8 with topical anti-glaucomatous medication.
We observed one case of mild keratopathy (Figure 1) in a patient treated with CCB plaque in 1997, which was stable over the years. The patient declined cataract surgery.
Twelve (50%) patients developed post-operative cataract in the median time of 38 months following treatment, and no case of bullous keratopathy post-cataract remo-val was observed. Five patients (21%) required temporary topical medications to control intra-ocular pressure (IOP), with the IOP rise in median post-surgery time of 14 months (range, 2-45 months). One patient (4%) developed chronic macular edema (CME) 19 months post-treatment, which was completely resolved with anti-VEGF therapy. The final outcomes regarding best corrected visual acuity (BCVA) are listed in Table 3. The final BCVA was between 1.0 and 0.5 for 16 (67%) patients, between 0.49-0.1 for 5 (21%) patients, and below 0.09 for 3 (12%) patients. Nine patients (37%) maintained initial visual acuity; it dropped more than 3 lines for 4 patients (17%) and improved in 6 patients (25%). No patient developed distant metastases by the end of the study.
Discussion
This was a single-center analysis evaluating long-term outcomes of ruthenium plaque treatment for iris melanoma. The major strength of our study is a long follow-up period of 67 months, which demonstrated good functional outcomes with standard shape of applicators used. The main weakness of the study is a relatively small number of studied patients.
Of note, due to low incidence of iris and iridociliary melanoma, there are few articles on this subject in the lite-rature, and most reported groups are not homogenous in terms of iridocorneal angle involvement. The median group size was 27 patients (range, 11-144 patients) [10]. The concept of irradiating iris tumor with a radioactive plaque is not new, it dates back to 1994 when Shields et al. presented a novel treatment method with 125I plaque for non-resectable iris melanoma adopted from posterior uveal melanoma management [13]. Since then, different types of plaques and radionuclides were used for irradiating iris tumors, showing satisfying outcomes [6, 11-18]. However, in a systematic review on plaque brachytherapy for iris and iridociliary melanoma by Karimi et al., there were only 12 papers included [10]. Different authors described the use of 125I, 103Pd, and 106Ru plaques in the management of iris melanomas [6, 11, 15, 17-21]. Various shapes of applicators were used, including round [15-17, 21, 22] and notched plaques [13, 16, 17]. The COMS plaques (125I, 103Pd) are utilized after a placement of amniotic membrane to protect the cornea. However, this is not necessary with ruthenium plaques.
Post-treatment cataract is the most common complication, and it affects 36-86% of all treated patients [10]. There has been a concern over the years of the corneal endothelium cell loss during the surgery, making the removal of the cataract somewhat a risky procedure. However, this concept has long been eliminated [23]; the cataract is normally removed safely with no major problems, and so was our experience. Nonetheless, 2 patients with the lowest BCVA in our group (below 0.09) were the ones who declined cataract removal.
Post-irradiation glaucoma is observed in 2-33% of all eyes treated with plaque placement [10]. In our group, this was well-controlled with topical therapy. The incidence of post-operative cataract and glaucoma in our study group are comparable to those found in the literature (Table 4).
Table 4
Post-treatment complications after use of different plaque radionuclides for iridociliary melanoma. Comparison of our results with results from the literature [6, 11, 14-18, 20]
| Complication | 106Ru | 125I | 103Pd | Our results |
|---|---|---|---|---|
| Post-treatment cataract (%) | 36-84 | 61-75 | 44-71 | 50 |
| Post-treatment glaucoma (%) | 36-84 | 17-33 | 2-29 | 21 |
| Local recurrence (%) | 0-5 | 0-8 | 0-4.1 | 4 |
The incidence of a local recurrence of post-plaque irradiation of an iris tumor is 0-8% [6, 15, 16, 18-20, 22] according to different reports. This can be very much dependent on the degree of angle involvement before the treatment, as some tumors tend to spread via the iridocorneal angle accounting for late recurrences.
We observed 2 quite rare complications, i.e., macular edema and superficial keratopathy (both single-case). They were also described by other authors with incidental occurrence [6, 16].
PBRT, as an alternative to plaque brachytherapy for iris and iridociliary melanoma management, has been used since 1993 and was first described by Damato et al. [24].
In terms of the incidence of 3 main post-operative complications, such as post-irradiation cataract, glaucoma, and local recurrence, the values are 20-47%, 3.4-31%, and 3.3-8%, respectively [24-26]. They do not diverge much from the results obtained with plaque therapy. Damato et al. presented the results of 155 iris melanomas treated with PBRT with a recurrence rate of 5.6%. Although it is comparable to previously mentioned results for plaque treatment, the authors emphasized that in such treatment, it is important not to underestimate tumor margins during the initial planning [27]. In our series, the use of 106Ru plaques of 2 sizes allowed to cover the tumor itself and the adjacent part of the ciliary body (or the whole segment with CCB plaque in diffuse cases), with the secured margins of the clinically visible tumor resulting in only one recurrence in the median time of 67.5 months. There has been some concern that PBRT might work better in diffuse iris melanoma cases, but if the whole anterior segment is irradiated, there might be a post-operative limbal stem cell deficiency leading to keratopathy [28]. This could be prevented by harvesting limbal stem cells before irradiation and subsequent autograft [29]. We did not observe such changes in our patients, and the only keratopathy in our group was presented as a line at the previously placed plaque margin.
Conclusions
Ruthenium-106 brachytherapy offers an effective way of treatment for iris and iridociliary melanoma, with a very low-rate of recurrence and acceptable level of maintained visual acuity in the treated eye. No major post-operative complications occurred in our study group during the long-term observation. The minor complications, such as post-irradiation cataract or rise of intraocular pressure, were easy to control and did not affect the final outcomes.



