Introduction
Mycosis fungoides (MF), a rare lymphoproliferative disorder characterized by accumulation of malignant T-cells in the skin, is the most common cutaneous T-cell lymphoma (CTCL) [1]. Pathophysiological mechanisms causing the progression of this disease have not been fully understood. A possible impact of tumour microenvironment on the development of the disease has been shown in some recent studies suggesting the impact of interleukin-17 (IL-17) in that matter [2]. A variety of therapies affecting cytokine profiles have been introduced, including tumour necrosis factor α (TNF-α)-inhibitors and TNF-α-receptor inhibitors (e.g. adalimumab, etanercept, infliximab), IL-17 and its receptor pathway blockers (bimekizumab, brodalumab, ixekizumab, secukinumab) and IL-12 and/or IL-23 pathway blockers (e.g. guselkumab, ixekizumab, risankizumab, secukinumab, tildrakizumab, and ustekinumab). They have been used more often in the clinical practice for the last decade.
Our aim in this review is to elucidate the role of IL-12, IL-17, IL-23 and TNF-α in MF, which sheds the light on the safety of new biologic treatments in psoriasis in context of CTCL.
Involvement of IL-17 in MF
The IL-17 family currently consists of six cytokines (named IL-17A to IL-17F) and five receptors (IL-17RA to IL-17RE) [3]. IL-17A, IL-17C and IL-17F are identified as proinflammatory, whereas IL-17E, also termed IL-25, is considered as anti-inflammatory [4]. IL-17A and IL-17F have 50% homology, therefore IL-17F signalling is 10–30-fold weaker [5]. IL-17A/17F heterodimer may also be secreted, and its signalling strength is intermediate [5].
IL-17A and IL-17F are secreted by many cells including Th17 cells, mast cells, macrophages, neutrophils and CD8+ lymphocytes [6]. The IL-17 cytokines are crucial in the answer against extracellular bacteria and fungi. When not strictly controlled, they can also contribute to development of pathogenic response causing autoimmune disorders such as psoriasis [7]. Possible involvement of IL-17 in the pathogenesis of MF has been reported by researchers [8–18].
In 2004 Cirée et al. were the first to reveal that tumour cells may express IL-17. The cells derived from patients diagnosed with MF or Sezary syndrome (SS) expressed IL-17 mRNA and secreted this cytokine in vitro [9]. Since then, several studies have reported conflicting results. We have found five studies, which have shown the elevated levels of IL-17 in biopsies from MF skin lesions [8, 11–13, 18]. IL-17F cytokine is known to induce gene expression of antimicrobial peptides (AMPs), other proinflammatory cytokines and matrix metalloproteinases (MMPs) [19] by activating numerous pathways such as NF-κB, MAPKs and C/EBPs [19]. Furthermore, IL-17F expression was associated with progressive CTCL [13]. Studies have also revealed that malignant lymphocytes in MF may present a Th-17 phenotype [11] which has been shown to express not only IL-17, but also IL-21, IL-22 and CCL20 [20, 21]. Two other studies have shown the results with normal levels of IL-17 in MF [10, 14]. One of them is suggesting that IL-22 rather than IL-17 is crucial in establishing the tumour microenvironment [10].
It is well known that activation of STAT3/JAK3 pathway plays a significant role in MF pathogenesis [11]. Krejsgaard et al. have found that malignant T-cells in CTCL not only can express IL-17, but also that expression is promoted by the JAK3/STAT3 pathway [11]. The-IL-17 pathway is important in innate defence mechanisms, promoting secretion of anti-microbial peptides (AMPs) or neutrophil-recruiting cytokines, thereby improving response against extracellular pathogens, e.g. Staphylococcus aureus [22]. Recently, it has been proven that T-cell receptor (TCR) engagement is necessary for malignant transformation in MF and that the progression of the disease is dependent also on microbiota [23]. The aetiology of MF is still elusive, nevertheless some bacterial agents are suspected to play an etiologic role. S. aureus has been one of them – reported to colonize 44–76% of CTCL patients [24–26] and to be the most common infection in CTCL [25]. Staphylococcal enterotoxin A (SEA) has been shown to stimulate activation of STAT3 and upregulate IL-17 production in primary patient-derived malignant and non-malignant T cells [16]. Researchers demonstrated the interesting cross-talk between malignant T-cells expressing SEA-nonresponsive TCR variable region β chain and non-malignant T-cell with SEA-responsive TCR, what may suggest that SEA-producing bacteria can promote the STAT3/JAK3 oncogenic pathway [16]. Furthermore, this may be the possible mechanism of the MF progression.
Despite those possible mechanisms and the elevated levels of IL-17 observed in MF patients [8, 11–13, 18], secretion of anti-microbial peptides is significantly lower in CTCL patients than in psoriatic skin. It may resemble what is seen in atopic dermatitis (AD) [14, 27], thereby suggesting the dysfunction in the induction of gene expression of antimicrobial peptides by IL-17, which is its role in healthy skin. Furthermore, studies seem to suggest the Th-2 cytokines to be the main players in advanced stages of MF and SS [28–30]. One study concerning IL-17E, called IL-25 here, showed that IL-25 levels are elevated in MF lesions and in sera of advance-stage patients, which also correlates with lactate dehydrogenase levels [18] known to reflect the CTCL activity [31]. What they concluded using MyLa cell lines (MF cell lines) was that IL-25 is secreted by epidermal keratinocytes in MF and may directly induce IL-13 secretion by tumour cells, which may contribute to formation of Th-2 microenvironment [18]. This mechanism seems to be relevant in the pathogenesis of MF and SS and may be clinically important since Geskin et al. established IL-13 to be an autocrine factor of CTCL, blocking of which may be the potential therapeutic target for clinical interventions [32].
Possible cancerogenic role of IL-17 in MF
Many studies have shown the link between IL-17 mediated answer and cancerogenesis, e.g. in colorectal cancer [33–37], lung cancer [38] and in squamous cell carcinoma (SCC) [39, 40].
A role of IL-17 in CTCL carcinogenesis has been still elusive to the best of our knowledge. Nevertheless, the pro-angiogenic role of IL-17 has been postulated in a few studies, therefore suggesting an indirect pro-carcinogenetic role. Along with the worsening survival rates as the stages of CTCL progress [1], some studies show that in the plaque and tumour stages, the value of the mean microvessel area is significantly higher [41, 42]. The promotion of angiogenesis is contributed to many factors and vascular endothelial growth factor (VEGF) has been one of them, a potent angiogenic protein, which is strongly induced by hypoxia [43]. The resistance of lymphomas, colorectal and lung cancer tumour models to treatment with anti-VEGF drugs, was promoted by Th17 subset cells [44]. VEGF has been present in CTCL lesions [45, 46] and its production is associated with constitutive activity of Janus kinase 3 (Jak3) and the c-Jun N-terminal kinases (JNKs) [45]. Moreover, Lauenborg et al. have shown that IL-17F secreted by malignant T-cell of MyLa2059 cell lines was able to trigger endothelial tube formation, thereby proving stimulation of angiogenesis by IL-17F [47]. Accordingly, when applying anti-cancer treatment, e.g. CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone) chemotherapy, IL-17 pathways may be indirectly promoted shifting to Th17 phenotype [17]. Similarly, promotion of Th17 pro-inflammatory cytokines has been noticed in colorectal carcinoma after administration of tamoxifen [37]. Moreover, IL-17RA blocking may be used to potentiate the response to such treatment [37]. Nevertheless, chemotherapy is not recommended as a first-line treatment for CTCL [48]. Mono- and polychemotherapy show elevated risks of death, no chemotherapy regimen has been shown to improve patients survival rates and remissions are significantly short [48]. In summary, IL-17 may have an impact on the promotion of carcinogenesis in MF and a possible aggravating role during chemotherapy. Despite some evidence of positive effects of blocking Th17 pathway during chemotherapy, for now, it is rather not a possible treatment in CTCL [48].
Role of IL-12/IL-23 in MF
Considering the impact of new biologic therapies on CTCL, it is important to mention the role of IL-12 and IL-23. Both cytokines, which share the same common subunit p40, are known to have pro-inflammatory effects [49]. Interestingly, despite having many similar functions, pre-Th-1 cells differentiate into Th-1 lymphocytes in the presence of IL-12, when IL-23 is present, they rather show Th-17 profile [50]. Though it is important to remember that Th-17 cells are easily interconvertible and they can be turned into Th-1 or Th-2 cells, when a suitable microenvironment occurs, but not vice versa [51]. Therefore recombinant IL-12, strongly inducing Th-1 microenvironment and with its ability to suppress Th-2 cytokines in SS, was considered as one of the emerging therapies for MF [52–54]. The main rationale for use of this cytokine in CTCL treatment was to enhance cell-mediated cytotoxicity and to restore interferon γ (IFN-γ) production [55], which expression has been shown to gradually decrease in the course of lymphoma progression [56]. IFN-γ has been used in MF treatment [57]. Phase II open-label study of recombinant human IL-12 in SS, which was administered in two subcutaneous injections per week in doses ranging from 100 ng/kg escalating up to 300 ng/kg, has shown some antitumor activity [58]. Another aspect is that IL-12 may be used as an early MF marker. Immunohistochemical staining of IL-12p35 has been shown to be useful as the diagnostic tool in patch-stage MF [59] along with exhibition of Th-1 dominant microenvironment in early stages of the disease [30]. The literature on IL-23 in MF is scarce. Due to the fact that IL-23 may induce pre-Th-1 cells to convert to Th-17, there is a possibility that this cytokine activates the secretion of IL-17A and IL-17F, thereby promoting the possible pathogenic effects of IL-17A and IL-17F. Doherty et al. have shown that there was an increased expression of IL-23 in keratinocytes and in dermal lymphocytes in all stages of MF, and that atypical lymphocytes infiltrating the tumour in IVB stage patients may demonstrate a weaker staining of IL-23 [60]. This finding shows that IL-23 may play a role in the pathogenesis of MF/SS, but further research is necessary.
Role of TNF-α in MF
TNF-α, the hallmark of pro-inflammatory cytokines, has also been shown to have an impact on MF. Studies seem to be consistent with the fact that TNF-α levels are elevated in the MF skin lesions [56, 61, 62]. Moreover, an increase in the cytokine concentration concomitant with the progression of the disease has been noticed [62] but not in all of cases [56]. What is also worth mentioning, serum concentrations of TNF-α in patients during treatment of CTCLs with extracorporeal photophoresis (ECP), have significantly increased from baseline during 6 months’ therapy, but no correlation with clinical response has been found [63]. Genetic studies suggest that patch-stage of MF is not determined by TNF-α genotype polymorphism [64]; however, Tracey et al. have found an association between tumorigenesis in MF and alteration in TNF receptor signalling [65]. They show the possibility of activating antiapoptotic pathways through first and second TNF receptors (TNFR1 and TNFR2) caused by deregulation of multiple genes, leading the lymphoma cells to avoid apoptosis, predominantly because of NF-κB upregulation [65]. Strong expression of these transcription factors may be one of the characteristic features presented by some MF cell lines [66]. Other researchers have shown that constitutive activation of NF-κB, pathway promoted by TNF-α, can cause the resistance to apoptosis in lymphoma HuT-78 cells [67]. TNF-α, alongside with IFN-γ, has been suspected to be one of the factors causing the epidermotropism in CTCL by inducing Interferon-Inducible Protein (IP-10) [56, 61]. Some of these latter reports were the basis to assess the treatment of a relapsed CTCL with soluble TNF receptor, etanercept [68]. Before the administration of the biologic drug, patients were heavily pretreated (median number of prior regimens was seven) [68]. This therapy was not effective in case of patients in the advanced stage of the disease: all of them did not respond and progressed [68]. Only two patients in the early stage of CTCL (both in IB), had some benefit from the therapy, one with partial response and the other with minor response [68], nevertheless this group is too small to make any reasonable conclusions.
MF, psoriasis and biological treatment implications
A significant pathogenetic role of IL-23, IL-17 and TNF-α in psoriasis is widely accepted and has important clinical implications in biological treatments of the disease. Anti-TNF-α drugs such as infliximab [69] and agents blocking IL-23, IL-17 pathway e.g. ustekinumab [70], ixekizumab [71] and secukinumab [72, 73] are more commonly used to treat chronic plaque psoriasis, often with superior therapeutic effects and increased quality of life [74, 75]. However, many studies have reported an increased risk of CTCL in patients with psoriasis both in Caucasian and Asian populations [76–78], especially in severe psoriasis [76, 78]. A major review on that topic also concluded that an association between MF and psoriasis is plausible, but prevalence and incidence have not been found yet [79]. Therefore, a question about the effect of these biologic therapies on the course of MF is raised. We believe that the most important issues here have been similar to those considered by Dequidt et al. [80]. First, if the biologic treatments in psoriasis may induce MF and second, in case of an overlap or misdiagnosis between those two diseases, if lymphoma may progress after administration of biologic drugs. Solely basing on the above assumptions, blocking IL-17RA (brodalumab), IL-17A (secukinumab, ixekizumab) and IL-17A-F (bimekizumab) may be beneficial in stopping progression of MF as we have shown the possible role of proinflammatory IL-17 cytokines. IL-17A and IL-17F may be included in progression of the disease by promoting the JAK3/STAT3 oncogenic pathway and by stimulation of angiogenesis. Furthermore, the anti-inflammatory cytokine, IL-17E, may contribute to creating a Th-2 dependent tumour microenvironment by inducing IL-13 secretion and brodalumab should be able to block this interaction. On the other hand, ustekinumab (blocker of p40 common subunit) and TNF-α inhibitors should not be recommended in the treatment of psoriasis if there is any risk of an overlap with CTCL as they are known to inhibit Th-1 microenvironment. Moreover, as it was shown, etanercept did not appear to be an effective drug in the treatment of CTCL, possibly causing the progression in some patients. Considering all the theoretical background, we have to focus on what researchers have found and published. Studies on the impact of anti-TNF-α drugs on the course of CTCL are much more abundant in contrast to the literature on other biologic therapies. We have identified 7 patients described in clinical case studies [81–85] and 90 cases from retrospective studies [80, 86–89] reporting CTCL after TNF-α-inhibitor treatment. Eighty-two out of these 97 patients presented with CTCLs, 66 of which were classified as MF and 5 as SS [80–89]. Dequidt et al. reported that in each of the 5 cases of large cell transformation in MF, the diagnosis of psoriasis was the reason to treat with biologic drugs and after discontinuing anti-TNF-α, the evolution of the lymphoma was aggressive [80]. Another study has revealed that the majority of MF were misdiagnosed, predominantly as psoriasis, and biologic drugs made the lymphoma fully apparent [87]. During follow-up, 7 patients died because of the CTCL that appeared after the anti-TNF-α administration, all of them in the advanced stage of the disease [86, 88, 89]. On the other hand, most cases of MF have appeared indolent after anti-TNF-α drugs were discontinued, in some cases the topical treatment led to partial or complete response [80, 89]. Moreover, majority of the patients had either a stable disease or a complete response after receiving a stage-suited therapy [80–89]. These results seem to be consistent with our previous considerations.
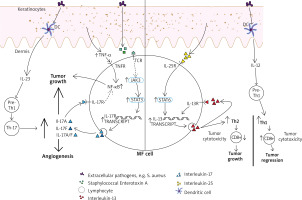
Figure 1
The contribution of interleukins (IL) 12, 17, 23 and tumour necrosis factor α (TNF-α) to the tumour microenvironment in mycosis fungoides (MF). IL-12 has been indirectly restoring the cytotoxic mediated CD8(+) answer and promoting tumour regression by stimulating the differentiation of pre-T-helper 1 lymphocytes. IL-23 can stimulate pre-T-helper 1 lymphocytes, followed by creating T-helper 17 cells subset and increased secretion of IL-17 proinflammatory cytokines. MF cell is also able to secrete IL-17A, IL-17F and IL-17A/IL-17F heterodimers. It is reinforced by upregulated JAK3/STAT3 pathway, which has been shown to be promoted by activated T-cell receptor (TCR), which is necessary for malignant transformation in MF to occur. One of the possible ways of activating TCR is related to Staphylococcal enterotoxin A. NF-kB upregulation, with its anti-apoptotic effect on lymphoma cells, seems to be important and relevant in the pathogenesis of CTCL. It has been promoted by TNF-α as well as proinflammatory IL-17 cytokines. IL-25 (IL-17E) is promoting STAT6 pathway. Those interactions result in increased IL-13 secretion (also in autocrine manner). Especially in the advanced stage of the disease it contributes to forming Th-2 cytokine profile, what results in decreased cytotoxic immunosurveillance and tumour growth
What may be surprising in terms of our IL-17 findings is what studies show. Most of the MF patients may progress after receiving IL-17A, IL-17RA or IL-12/23 inhibitors [80–82, 89, 90]. In fact, some case report has shown significant clinical improvements after discontinuing of these drugs [81]. Nevertheless, the biggest study on that matter has shown that in 8 of 11 cases, a worsening of the disease was noticed and in the short follow-up of thirteen months 5 patients died, 4 of MF and one of stroke [89]. In contrast to these reports, our assumptions highlighted the possible aggravating role of IL-17 in MF, therefore blocking it would be beneficial. The explanation to these conflicting data may be the aspect of Th17/Treg imbalance leading to immunosuppression [90] and other, not yet known mechanisms. Admittedly, despite the fact that the literature is scarce on the effects of these newest biologic therapies on MF, it seems to be ethically questionable to make further intentional research elucidating these aspects. Also, many authors emphasized what may be done in order to minimize the risk of a lymphoma progression after receiving biological drugs. The most important conclusion is to carefully examine patients and in case of any oncological suspicion, take biopsies in order to exclude a potential misdiagnosis [80, 82–86, 89, 90].
The main limitation of the study in the aspect of biologic therapies is based on the retrospective studies and case reports. The disturbing role of some other medicaments, like cyclosporine, also cannot be excluded, since most patients with psoriasis have undergone at least one non-biological systemic therapy before receiving biologic drugs.
Conclusions
Interleukin-17 is detectable in MF lesions, sometimes with the elevated level, but it does not seem to be the main player of MF. We show it not to be as important in the pathogenesis of CTCLs as it is in the pathogenesis of psoriasis, nevertheless using IL-17 or IL-17RA blockers (bimekizumab, brodalumab, ixekizumab, secukinumab) may cause a progression of MF in case of an overlap or a misdiagnosis of the mentioned autoimmune disease. Based on the literature, we have also described the beneficial effects of IL-12 on MF, therefore the agents blocking both IL-12/IL-23 pathway (ustekinumab) should be avoided in patients, who have a suspicion or a diagnosed MF. Lastly, the overall contribution of TNF-α to creating cell mediated cytotoxic Th1 microenvironment seems to outweigh the negative effects of TNF-α on the lymphoma, which was reported. TNF-α-inhibitors and TNF-α-receptor inhibitors (e.g. adalimumab, etanercept, infliximab) should not be used if CTCL cannot be ruled out. Before introducing the biological treatment, in case of advanced to severe psoriasis, we recommend performing a biopsy from the skin lesion followed up by a close pathological examination to exclude the possibility of MF misdiagnosis. We believe that further research is necessary to clarify the role of IL-17, IL-23 and TNF-α in MF and new immunosuppressive drugs should be used carefully in order not to aggravate the plausible lymphoma.








