Introduction
Patients with type 1 diabetes are at high risk for early skin microcirculatory impairment [1–3]. Its functional impairment, which precedes structural pathology, may be detected with the use of venous occlusion (VO) or post-occlusive reactive hyperaemia (PORH) tests, both of which involve shear stress and its consequences [4–7]. Microvascular function using capillary recruitment during PORH is secondary to ischaemia and related to endothelium-dependent vasodilatation at the level of precapillaries mediated by an axonal reflex and the endothelium derived hyperpolarizing factor [8, 9].
The influence of these provocation tests on tissue oxygenation may be assessed with transcutaneous oxygen pressure (tcPO2) measurements, which also allow for simultaneous functional evaluation of skin microcirculation. In recent years, the practical value of tcPO2 in screening and follow up of overt vascular disease has been well documented [10–16]. TcPO2 values have been also confirmed as an independent prognostic marker for 1-year mortality among patients with type 1 diabetes and diabetic foot ulcers [17]. However, despite its high diagnostic value, tcPO2 measurements have not been recently reported in younger populations with less advanced skin microangiopathy. The influence of lipid and thyroid hormone levels on tcPO2 also remains unclear.
Aim
Our aim was to compare tcPO2 in children with short-lasting non-complicated type 1 diabetes mellitus (DM1) and age-matched healthy controls with regard to serum lipid and thyroid hormone concentrations.
Material and methods
The study group consisted of two subgroups: 51 paediatric patients with type 1 diabetes (median duration: 4.7 (1.2–9.9) years, median age at onset: 10.4 (2.1–13.9) years, and 28 age-matched control subjects (Table 1). Patients were recruited from the Department of Paediatrics, Diabetology and Endocrinology at Medical University of Gdansk in 2014–2018. Only patients with short-lasting (disease duration of less than 10 years) diabetes without clinical evidence of micro- or macroangiopathic complications of type 1 diabetes were included in the study. The only medication the patients were using was insulin, either through a pump or a pen. Based on medical history, physical examination and biochemical analysis, none of the study subjects had any form of microangiopathy, including retinopathy, nephropathy or neuropathy. All examinations were performed between 8:00 A.M. and 1:00 P.M. The study protocol included medical history, tcPO2 and laboratory testing. The lack of microangiopathy (retinopathy, nephropathy and neuropathy) was confirmed using previously published criteria [18–20]. The patients from all study groups were euthyroid at the time of investigation.
Table 1
Characteristics of study groups
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the Medical Ethics Committee of the Medical University of Gdansk, Poland and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Medical Ethics Committee of the Medical University of Gdansk (NKBBN/277/2014; NKBBN/277-512/2016). Upon entering the study each participant has given informed consent. Parents agreed to carry out microcirculation tests and they were present during the tests.
Transcutaneous oxygen pressure examination
During the examination, patients remained in a comfortable sitting position with the upper limb freely supported at the heart level. Core body temperature was controlled with the use of a contactless thermometer (Novama – Model NT 19) and was within normal range in all examined patients and control subjects. The room temperature was controlled by air conditioning and was kept the same during all tests.
Transcutaneous oxygen pressure examination was assessed with PeriFlux System 5000 (Perimed AB, Sweden). The level of tcPO2 is based on the amount of oxygen that diffuses from the capillaries through the epidermis to the electrode and thus provides information about the body’s ability to deliver oxygen to tissues [18]. In the tcPO2 measurement, sensors that contain a pair of polarized electrodes are used, enabling the determination of oxygen content in a given volume. The classic oxygen electrode contains a silver chloride anode and a cathode made of gold or platinum, separated with liquid electrolytes. Electrodes are bound by a polymer membrane (Teflon, polyethylene or silicone polymer), which selectively permeates oxygen from the examined area of the skin [21]. The output current is proportional to the partial pressure of oxygen in tissues [22] and skin circulation, oxyhemoglobin dissociation and tissue metabolic activity. An electrochemical electrode and a local heating system were used for the measurements. In PeriFlux System 5000 it is possible to heat up the electrode to a temperature range of 37–45°C. The skin temperature of 43°C was chosen for the purpose of achieving local vascular congestion. A comparable temperature was used in a study by de Meijer [23] and was proposed in “The tcPO2 handbook” [24].
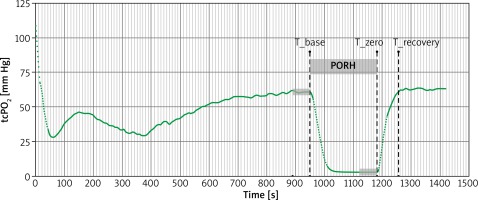
After calibrating the device and heating the Clarke-type electrode to 43°C, the electrode was placed in the adhesive groove on the central part of the hairless forearm. During the PORH test, the blood pressure cuff was placed around the patient’s arm and inflated to a pressure 50 mm Hg greater than systolic blood pressure for 4 min [25]. TcPO2 was continuously recorded during rest (baseline), occlusion (PORH) and after cuff release (recovery period) (Figure 1).
The curve analysis included identification of specific time points i.e. T_base as the moment of initiation of occlusion, T_zero as the moment of end of occlusion and T_recovery as the moment when pre-occlusion value was observed again.
The following parameters were determined:
Statistical analysis
All the analyses were performed using Statistica data analysis software system, version 12 (StatSoft, Inc., Tulsa, OK, USA). Shapiro-Wilk tests were performed to analyse the distribution of continuous variables. Values are expressed either as median and range or as mean and standard deviation, as appropriate. For group’s changes, Wilcoxon test was used. The comparison between groups was performed using the Mann-Whitney test. The χ2 test was used to compare the proportion of genders and the frequency of mild and severe hypoglycaemia. The comparison between groups with regard to associated continuous variables was performed using the General Linear Model with Fisher test for post-hoc analysis when needed, multivariate analyses were performed to assess the independent impact of biochemical parameters on skin oxygenation. The value of p < 0.05 was deemed statistically significant.
Results
Transcutaneous oxygen pressure at maximal ischemia (tcPO2_zero) was higher in children with type 1 diabetes than in healthy controls (p = 0.002). There were no differences between groups regarding tcPO2 prior to PORH initiation (tcPO2_base) or tcPO2 at recovery after PORH (tcPO2_diff and TTR) (Table 2). Serum concentrations of total cholesterol, triglycerides, HbA1c and TSH were higher, and fT4 were lower in children with type 1 diabetes than in healthy controls. No other differences between the groups were found (Table 1). There was no significant impact of gender on the tcPO2 parameters.
Table 2
Characteristics of tcPO2 parameters in studied groups
After adjusting for total cholesterol and triglycerides levels, the differences in tcPO2 between type 1 diabetic group and controls were no longer observed (Table 2). An analysis of the collected data showed no correlation between the age at type 1 diabetes onset, duration of the disease, insulin dose, treatment with pump, HbA1c level and tcPO2 parameters in the diabetic group. There were no correlations between serum concentrations of total cholesterol, triglycerides, TSH and ft4. Multivariate analysis showed an impact of total cholesterol on tcPO2_zero in DM1 patients, but not in healthy controls (Table 3).
Discussion
In this study, we have observed an impairment in skin oxygenation reflected by a significant difference in the measured tcPO2 parameters between the diabetic and control groups. Adjustments for total cholesterol and triglycerides have eliminated the differences in the skin oxygenation between the groups. To the best of our knowledge, the effects of lipids on transcutaneous oxygen pressure have not been previously described in the literature. Due to the fact that lipids have an effect on endothelial function, thus impacting microcirculation [26–28], we assumed that they may influence tcPO2 as well.
Hypercholesterolemia is associated with significant retinal microvascular dysfunction as evidenced by a reduction in flicker-induced dilatation of retinal arterioles [29]. Elevated low-density lipoprotein (LDL) cholesterol and triglycerides, lower high-density lipoprotein (HDL) cholesterol as well as older age, duration of diabetes, smoking, increased diastolic blood pressure and obesity, are all risk factors for peripheral neuropathy in type 1 diabetes [30]. Among adolescents with type 1 diabetes, the use of a statin did not change the albumin-to-creatinine ratio over time although it resulted in significant reductions in total, low-density lipoprotein and in triglyceride levels [31]. Research data show that the impaired skin microcirculation response can be reversed after cholesterol-lowering therapy [32, 33]. It is worth noticing that in both studied groups several subjects had extremely low tcPO2_base values as it was measured in the forearm. These values were even lower than the threshold established for diagnosis of critical limb ischemia. However, direct comparison of these values may be misleading because of different electrode placement.
Studies of some authors have shown that tcPO2 is reduced in diabetes. These results coincide with the co-occurrence of neuropathy and may precede its appearance. However, these reports come from the 1980s and 1990s [34–37]. During that time, less effective prevention, treatment and care could have been responsible for the observed changes in microcirculation even after a short duration of diabetes. Ewald’s study shows an abnormal hyperaemic response in 13 diabetic children [34]. Skin postocclusive hyperaemia was recorded with a transcutaneous oxygen electrode at 37°C on admission before introducing insulin treatment and 1, 6, 12 and 24 months after diagnosis. To achieve congestion they used different temperatures.
It appeared that after diabetes has been present for 2 years, the vascular reactivity was significantly lower than in the control group. Breuer et al. designed a study in which they have examined 76 type 1 diabetic patients with disease duration ranging from several weeks to 30 years (median: 6.0 years) and mean HbA1c of 8.9%. They showed reduced transcutaneous oxygen pressure associated with type 1 diabetes mellitus. This relationship was observed even in patients who at the time of the study showed no signs of micro- or macroangiopathy. The dependence of this phenomenon on the duration of diabetes was not revealed [35]. De Meijer et al. [23] reported that 60 adult type 1 and type 2 diabetic patients without signs of neuropathy had significantly decreased tcPO2 values. They suggested tcPO2 measurements in diabetic patients could be useful to detect subclinical microvascular impairment. In this study, there was no difference between the type 1 diabetes and control groups. When type 1 and type 2 diabetic patients were combined into one group, significant differences were found between the tcPO2 values in the diabetic and non-diabetic groups.
Our study analysis of the influence of gender on tcPO2 parameters did not show any differences either in the diabetes or the control group. Similar results were also obtained by Rodrigues who compared tcPO2 between groups of adult men and women [37]. Conversely, a study by Orenstein et al. on healthy volunteers showed that women had had significantly higher tcPO2 than men [38]. In Lagerkvist’s study, the tcPO2 value obtained in healthy children and adolescents was 81 (65–97) mm Hg [39], while in our study the tcPO2 value recorded on the arms of control subjects at baseline was 56.3 (18.6–81.1) mm Hg. The difference between the obtained results can be explained by different electrode placement and higher temperature used in Lagerkvist’s study. In their study, patients were examined in a sitting position, with their left elbow joint in 90° flexion and with their forearm resting horizontally on a pillow placed on their knees.
Most of the studies available in the literature concern adult patients with type 2 diabetes. Our study was specifically designed to be performed on children with type 1 diabetes with no clinically apparent microangiopathic complications in order to find other evidence of early deterioration of microcirculation. In the group of patients with diabetes, tcPO2_zero was significantly higher. Lack of data in the literature makes it impossible to refer to this parameter. It appears that this parameter indicates the efficiency of oxygen perfusion through tissues, i.e. skin flow. A higher tcPO2_zero value indicates poorer skin microcirculation efficiency. The high-cholesterol levels adversely affect microvasculature. Hypercholesterolemia impairs endothelium dependent vasodilation because of defects in nitric oxide bioavailability. The pathogenic mechanism underlying microvascular dysfunction involves many steps that consequently lead to induction of inflammation and a prothrombotic phenotype [40]. After adjusting for in total cholesterol and triglyceride levels, skin flow disorders have been modified. Hence, it appears that the use of pharmacological intervention at an early stage of lipid disorders is able to reverse early impairments in skin microcirculation.
The limitation of our study may be the imbalance between the subgroup numbers, which could potentially influence the power of the study. Additionally, discussion of the results we have obtained is difficult due to the lack of other reports on patients with short-standing diabetes tested in a similar manner. However, our work brings a lot of new data into the discussion on transcutaneous oxygen pressure measurement as it was carried out on a large group of 79 children. So far, the studies with the highest numbers of participants have examined 158 individuals, only 76 of which were diabetic patients [35]. The projects in which children were studied were even less numerous [39].
The natural conclusion from the results obtained is the fact that it would be extremely important to analyse the parameters of transcutaneous oxygen pressure after treatment with statins due to reports of the protective nature of statins in other vascular areas [32, 33]. It seems that such a study could confirm our theoretical considerations in practice. Finally, we conclude that increased lipid levels are responsible for impairment of microvascular skin response to ischemic stimuli in short-lasting type 1 diabetes. Lipid-lowering therapy in children with type 1 diabetes, even in the absence of clinically evident microangiopathy, may be considered beneficial for skin microcirculation.