Introduction
Frontal fibrosing alopecia (FFA) is primary lymphocytic cicatricial alopecia characterized by a progressive course and permanent damage to hair follicles. The disease was first described by Kossard in 1994. Progressive cicatrization moves the frontotemporal hairline backwards. The eyebrow hair is lost in 50–85% of cases (the external part of the eyebrows in one-third of cases and the entire eyebrows at an advanced stage); some patients lose their body hair. The disease usually occurs in postmenopausal women.
In accordance with scientific knowledge, we assumed that FFA is presently considered as a subtype of lichen planopilaris (LPP). We intend to continue the study on a larger study group, then we intend to separate these 2 units.
FFA and LPP comprise a disease consisting of progressive and irreversible hair loss due to hair follicle damage by an autoimmune process. It typically begins with red and violet papules or pink areas accompanied by scalp exfoliation. The lesions are located mainly in the parietal region and the head top. A frequent coexisting symptom is pruritus and hyperesthesia of the affected skin. The disease is usually progressive, but its activity remains variable. LPP treatment strategies depend on disease severity, the patient’s age and preferences, as well as the physician’s experience [1, 2]. As with all the types of cicatricial alopecia, the primary aim of treatment is alleviation of symptoms and cessation of the cicatrization process. The first-line treatment of LPP and FFA involves topical corticosteroids. Other suggested therapies include oral administration of prednisone, retinoids, cyclosporin, mycophenolate mofetil, azathioprine, thalidomide, tetracycline, and griseofulvin [3–6]. Systemic treatment is often necessary due to intense symptoms accompanying the disease such as burning, pruritus, and hypersensitivity of the scalp. Those symptoms are the disease activity markers and frequently correlate with irregularities in the trichoscopic image. Drugs modifying the course of that disease, including hydroxychloroquine, increasingly often supplement its therapeutic portfolio.
Hydroxychloroquine plays an important role in dermatological treatment owing to its anti-inflammatory properties. It has been used since the 1950s. It rarely interacts with other preparations and has a relatively low toxicity. Therefore, the application of antimalarial drugs allows one to reduce the therapy with, among others, glucocorticosteroids.
Aim
The study described herein was aimed at assessing the efficacy of hydroxychloroquine in the treatment of LPP and FFA.
Material and methods
The retrospective analysis included 95 women (average age: 57) (Table 1), including 35 with FFA and 60 with LPP (Table 2). The diagnosis was based on clinical morphology and in most cases was backed up with a histopathological examination (in 84 out of 95 subjects).
Table 1
The basic statistics concerning the patients’ age and disease duration before therapy commencement. Source: own work (n = 95) (n – sample size)
| Variable | Mean | Standard deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| Age [years] | 57.68 | 9.93 | 61 | 29 | 72 |
| Follow-up time [years] | 4.83 | 3.69 | 4 | 0 | 8 |
The medical documentation was reviewed in terms of disease intensity and follow-up periods. All the patients underwent ophthalmological consultation before starting the drug as well as after 6 and 12 months of the therapy. They also underwent complete blood count and liver function tests before the therapy and then every 3 months.
All the patients were treated with hydroxychloroquine for 1 year (dose: 200 mg/day, 5 days a week excluding weekends). The Saturday-Sunday break is to minimize the occurrence of side effects resulting from the treatment. This scheme is used in the clinic where the study was performed, and many years of experience show that this helps to reduce the incidence of side effects. The follow-up was conducted in the years 2010–2018 in the Department of Dermatology at the Medical University of Silesia in Katowice, Poland (Table 3). The average time needed from making a diagnosis to commencing the therapy exceeded 4 years (Table 1). The patients were monitored for disease progression throughout the 3 months after discontinuing the systemic treatment.
Table 3
The number of patients whose diagnosis was established in the particular years. Source: own work (n = 95)
| Year | Number of patients |
|---|---|
| 2010 | 4 |
| 2011 | 6 |
| 2012 | 8 |
| 2013 | 11 |
| 2014 | 11 |
| 2015 | 17 |
| 2016 | 27 |
| 2017 | 9 |
| 2018 | 1 |
| Total | 94 |
| No data | 1 |
The response before and after hydroxychloroquine treatment was analysed using the LPP activity index (LPPAI). The LPPAI values were calculated before we started treatment and after 6 and 12 months. LPPAI is a numerical score used to assess LPP and FFA intensity and perform a statistical comparison; it was introduced by Chiang et al. [7]. The LPPAI algorithm was confirmed in terms of correlation with the clinical response in earlier studies [8]. The index values range from 0 (no evidence of a clinically active disease) to 10 (the highest activity).
The index includes 8 subjective (symptoms) and objective (signs) markers of disease activity. The markers received numerical values enabling the determination of disease activity. The symptoms are pruritus, pain, and burning (30%), while the signs are monitored by trichoscopy and include redness, perifollicular erythema, and perifollicular keratosis (30%). The remaining markers are the pull test (25%) and spreading of the disease (15%).
The index was created by weighing every criterion according to its reproducibility and objectiveness. For example, the pull test is the most reproducible and objective of all the criteria, so it received the highest weight (×2.5). Spreading of the disease received a lower weight (×1.5) because it is a subjective or less reproducible marker. Pruritus, pain, burning, and trichoscopic signs (redness, perifollicular erythema, and perifollicular keratosis) were measured using the following scale: 0 – absent; 1 – mild; 2 – moderate; 3 – severe. The pull test can be negative (0) or positive (1). The spreading of the disease was measured using the following scale: 0 – no spreading; 1 – indeterminate; 2 – spreading. The LPPAI values were calculated using the following equation: LPPAI = (pruritus + pain + burning)/3 + (redness + perifollicular erythema + perifollicular keratosis)/3 + 2.5 × pull test + 1.5 ´ (spreading of the disease/2).
The LPPAI scores were calculated at the beginning of the therapy with hydroxychloroquine as well as after 6 and 12 months of the therapy. A disease activity decrease reflected by a lower LPPAI (a reduction by 25–85%) was considered as a therapeutic success. The treatment was deemed to have failed when LPPAI lowered by < 25%.
Statistical analysis
The material obtained from questionnaires (Table 4) filled in by physicians was encoded in a database created using Microsoft® Excel. Every trichoscopic examination was performed by two researchers to minimize the systematic error of measurement. Then, the data underwent a statistical analysis using StatSoft® Statistica 13.
Table 4
Lichen planopilaris activity index. Source: the table – own work
The goodness of fit for the distribution of continuous quantitative variables in comparison with normal distribution was assessed using the Shapiro-Wilk test. Where the goodness of fit was established for the distribution of attributes in comparison with normal distribution, we analysed the correlation between quantitative variables using the t-test of dependent samples. Where the sample distribution for an analysed attribute significantly deviated from normal distribution, we applied a nonparametric test: the Mann-Whitney U test. To analyse the differences and dependences concerning qualitative variables we applied the χ2 test of independence or Fisher’s exact test. The significance level assumed for the performed statistical analyses was p ≤ 0.05.
Results
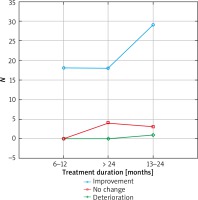
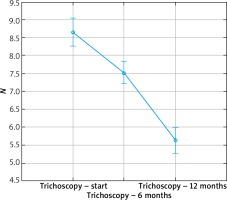
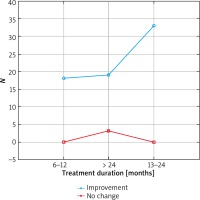
The results were presented in Figures 1–3. In the 6th month of treatment, 58 subjects showed a disease activity decrease (LPPAI reduced by 25–85% in comparison with the baseline value), 19 subjects achieved no improvement (LPPAI reduction by < 25%), and 3 subjects noted a deterioration (an LPPAI increase). After 12 months of treatment, most (70 out of 73) patients showed an LPPAI improvement (Table 5). Treatment with hydroxychloroquine has a statistically significant effect on decreasing the disease activity after 6 (Figure 4) and 12 (Figure 5) months of therapy. The statistical test results did not confirm the existence of a correlation between the age range and treatment efficacy between the 6th and 12th month or after 12 months of pharmacotherapy (Figures 6, 7). However, they confirmed the existence of a correlation between therapy duration in months and treatment efficacy (p < 0.05) (Figures 8–10).
Table 5
Therapy effects visible in the trichoscopic image 6 and 12 months after starting the pharmacological treatment. Source: own work (n = 95)
| Examination time | Number of examined patients | Improvement | No changes | Deterioration |
|---|---|---|---|---|
| Before the therapy | 95 | |||
| After 6 months of therapy | 80 | 58 | 19 | 3 |
| After 12 months of therapy | 73 | 70 | 3 | 0 |
Figure 2
Perifollicular keratosis and perifollicular erythema: before (A) and after (B) the treatment with hydroxychloroquine
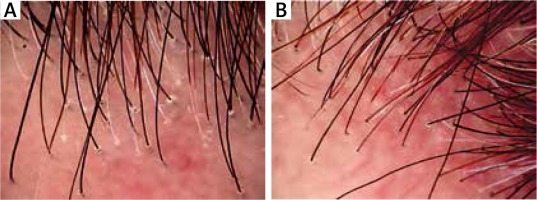
Figure 3
Corneous collars in lichen planopilaris: before (A) and after (B) the treatment with hydroxychloroquine
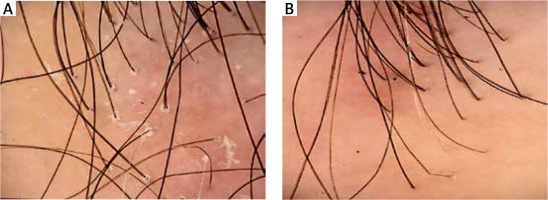
Figure 4
Box plot for the t-test of dependent samples: a comparison of trichoscopy results before and 6 months after starting the pharmacotherapy. Source: own work (n = 80)
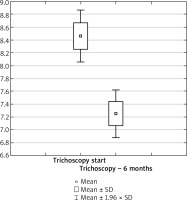
Figure 5
Box plot for the t-test of dependent samples: a comparison of trichoscopy results before and 12 months after starting the pharmacotherapy. Source: own work (n = 73)
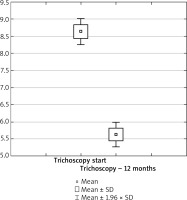
Figure 6
Treatment efficacy visible in trichoscopy results between the 6th and 12th month of pharmacotherapy depending on the patient’s age range. Source: own work (n = 73)
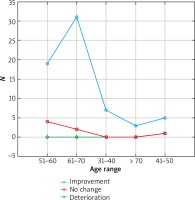
Figure 7
Treatment efficacy visible in trichoscopy results after 12 months of pharmacotherapy depending on the patient’s age range. Source: own work (n = 73)
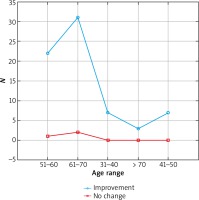
Figure 8
Treatment efficacy visible in trichoscopy results after 12 months of pharmacotherapy depending on the treatment duration. Source: own work (n = 73)
Discussion
Cicatricial alopecia is diagnosed less often in clinical practice than non-cicatricial alopecia types, and the differential diagnosis of cicatrization types is frequently difficult [4–6]. In doubtful cases, trichoscopic and histopathological examinations of a scalp specimen may be helpful in making a correct diagnosis.
The study described herein concerned lesions visible in the trichoscopic images of patients with FFA and classic LPP.
Active FFA typically involves slight perifollicular keratosis and prevalence of single-hair follicles, especially near the hairline. A more frequent presence of dendritic vessels has been confirmed by one study [9], but it is not a pathognomonic feature for this diagnosis [10–12].
LPP is the most frequent cause of primary cicatricial alopecia in adults. It is divided into 3 types: classic LPP , frontal fibrosing alopecia, and Graham-Little syndrome [13].
The most characteristic trichoscopic features of classic LPP are perifollicular keratosis, perifollicular inflammation, violet intercellular spaces, and elongated blood vessels [10–12]. Perifollicular keratosis is characteristic for the active form of this disease. The scales migrate along the hair shafts and form tubular structures that cover the proximal parts of the growing hair shafts. Those are called collar-like structures or tubular perifollicular keratosis. The hair shafts may be covered with such scales up to several millimetres above the skin surface.
A review of the available literature has not revealed randomized controlled trials concerning the therapy of frontal fibrosing alopecia. The literature mentions 114 patients diagnosed with FFA, who received 10 different therapeutic strategies. The most frequent of these was oral administration of 5α-reductase inhibitors, which produced a good clinical response in 45% of the subjects. Hydroxychloroquine caused a good clinical response in 30% of the 29 treated patients. Topical corticosteroids proved inefficacious in FFA. The remaining therapies were administered to less than 10 patients [14].
The reports on the benefits of administering hydroxychloroquine in cicatricial alopecia are mixed, but a study conducted by Samrao et al. noted a significant reduction of the signs and symptoms during and after the treatment [15].
The low number of patients diagnosed with FFA who were treated in other ways (tetracycline, triamcinolone, or UVB phototherapy) excludes any conclusions regarding the efficacy of those methods. Mycophenolate mofetil was recently added to the therapeutic spectrum of FFA treatment [16].
A study conducted in the years 2007–2017 assessed the efficacy of systemic retinoids in FFA treatment. The retrospective analysis involved 54 women with FFA treated with the following: oral isotretinoin – daily dose: 20 mg (29/54); acitretin – daily dose: 20 mg (11/54); and oral finasteride – daily dose: 5 mg (14/54). The treatment with systemic retinoids lasted 12–16 months (average time: 13.5 months). The primary aim of treatment was defined as lack of disease progression after 12 months of therapy, while the secondary aim was a lack of disease progression after the discontinuation of systemic retinoids. The primary aim of treatment was achieved by 76% (23/29) of the patients treated with isotretinoin, 73% (8/11) of the patients treated with acitretin, and 43% (6/14) of the patients treated with finasteride. The secondary aim of treatment was achieved by 72% (21/29) of the patients treated with isotretinoin, 73% (8/11) of the patients treated with acitretin, and 43% (6/14) of the patients treated with finasteride. Consequently, systemic retinoids may be beneficial for frontal hairline stabilization [17].
Two biological drugs, rituximab (an anti-CD20 monoclonal antibody) and pioglitazone (a PPAR-γ agonist), were described in 2 cases [18, 19] of treating LPP patients. Those biological drugs yielded promising results, probably due to their selective targeting on the pro-inflammatory particles in the lymphocytic infiltration. Pioglitazone was recently studied in a group of 24 patients with resistant LPP, causing disease remission in 5 (20%) of the subjects and a partial improvement in 12 (50%) others [20]. The effect of pioglitazone depended on its dose.
The presently available literature contains no unambiguous guidelines on FFA or LPP treatment, which challenges clinicians to seek the best treatment strategy for those diseases. In many cases, moderate and strong topical corticosteroids are insufficient [14].
The retrospective analysis performed in the present study has shown that hydroxychloroquine seems an efficacious drug in the inhibition of FFA and LPP progression and should be considered as first-line treatment for those diseases. After 12 months of treatment, most (70 out of 73) subjects showed an improvement of their trichoscopic image.
Oral antimalarial drugs are viewed as one of the therapeutic options available in this case. Hydroxychloroquine efficacy was analysed in 16 patients with FFA in a certain retrospective study. The percentage of patients responding to the treatment reached 36% after 6 months of therapy and 62% after 12 months [15].
In 2010, Chianget et al. followed up the responses before and after hydroxychloroquine treatment in 40 patients with LPP, including 11 with FFA. The data showed a statistically significant reduction of perifollicular keratosis after 6 months and a further significant reduction after 12 months.
Our data indicate that hydroxychloroquine significantly improves the trichoscopic image in FFA and LPP patients after 6 and 12 months of treatment (p < 0.05). The maximum benefits of hydroxychloroquine administration are visible within 12 months of therapy. Our own follow-up indicates that long-term therapy seems a more beneficial option.
As an antimalarial drug, hydroxychloroquine causes the following adverse reactions: gastrointestinal symptoms (nausea, vomiting, diarrhoea), neuromuscular symptoms (headache, muscle pain, fatigue), and skin hyperpigmentation from blueish grey to black, which subsides after therapy completion. Patients with glucose-6-phosphate deficiency and late skin porphyria (porphyria cutanea tarda) may develop haemolysis and acute hepatitis, respectively. The drug may also cause reversible leukopenia. Due to the observed affinity of antimalarial drugs to pigments, including melanin, the treatment may increase the risk of retinopathy development. The recommendations by the American Academy of Ophthalmology concerning screening assays during hydroxychloroquine administration, which were published in 2002, include a basic retina examination every 6 to 12 months [21, 22]. A large retrospective study involving 1027 patients did not reveal any toxic action of hydroxychloroquine [23].
None of the patients taking the drug during that study developed a serious adverse event, including retinopathy. The adverse reactions reported by the patients included the following (in descending order): increased gastric symptoms (n = 16), vision disturbances (n = 9), discolorations (n = 3), skin pruritus (n = 3), muscle power decrease/muscle pain (n = 2), vertigo (n = 1), headache (n = 1), urticaria (n = 2), and elevated liver enzymes (n = 1). Only in 2 cases was the treatment discontinued due to urticaria. Most adverse reactions were reported up to 3 months after the hydroxychloroquine therapy initiation.
Conclusions
Administration of hydroxychloroquine can be considered as one of the treatment methods for FFA and LPP in everyday clinical practice.
The presented study is the first attempt at using hydroxychloroquine to treat large patient groups for FFA and LPP. Hydroxychloroquine efficaciously alleviates the symptoms in LPP and FFA patients, gives maximum benefits in a long-term therapy (12 months), and effectively inhibits disease progression.
The preliminary results obtained in the presented retrospective analysis should be confirmed in a randomized prospective clinical trial, which remains a future challenge for researchers.