Introduction
Asthma is well known as a serious public health problem in the world [1–3]. It is a heterogeneous disease and considerable progress and distinct phenotypes may respond differently to treatment [4–6]. During the active airway inflammation, the leukocytic infiltrate can be eosinophilic or neutrophilic or mixed. 40–50% of asthma patients are estimated to have eosinophil-dominant airway inflammation [7, 8]. The chronic inflammatory process mediated and orchestrated by products of certain immune cells leads to the clinical manifestations in these patients [9–13].
CRTH2 is a G-protein-coupled receptor expressed by Th2 lymphocytes, eosinophils and basophils. It has a dominant role in the activation of these cell types responding to the mast cell product prostaglandin D2 (PGD2), which is followed by increased cell migration and elaboration of Th2 cytokines [14–16]. CRTH2-deficient mice or animals with CRTH2 antagonists revealed the involvement of this receptor in effector responses to allergens including leucocyte accumulation, Th2 cytokine production, tissue swelling, airway hyperresponsiveness and production of IgE [17].
CRTH2 antagonist OC 000459 was documented to reduce airway inflammation, improve lung function and quality of life in moderate persistent asthma [18, 19]. Several RCTs have explored the efficacy of CRTH2 antagonist OC 000459 for the asthma control, but the results were inconsistent [19–21].
Aim
We therefore conducted a systematic review and meta-analysis of RCTs to evaluate the effectiveness of CRTH2 antagonist OC 000459 on asthma control.
Material and methods
This systematic review and meta-analysis were conducted according to the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement [22] and the Cochrane Handbook for Systematic Reviews of Interventions [23]. All analyses were based on previously published studies, and thus no ethical approval and patient consent were required [24].
Literature search and selection criteria
PubMed, EMbase, Web of science, EBSCO, and the Cochrane library were systematically searched from inception to September 2019, with the following key words: “CRTH2 antagonist” or “OC 000459”, and “asthma”. To include additional eligible studies, the reference lists of retrieved studies and relevant reviews were also hand-searched and the above process was performed repeatedly until no further article was identified.
The inclusion criteria were as follows: (1) patients were diagnosed with chronic asthma; (2) intervention treatments were CRTH2 antagonist OC 000459 versus placebo; (3) the study design was RCT.
Data extraction and outcome measures
The following information was extracted for the included RCTs: first author, publication year, sample size, forced expiratory volume in 1 s (FEV1), Predicted FEV1 and detailed methods in two groups, etc. The author would be contacted to acquire the data when necessary.
The primary outcomes were FEV1 change, and predicted FEV1 change. Secondary outcomes included peak expiratory flow change, respiratory tract infection and treatment-related adverse events.
Quality assessment in individual studies
The Jadad Scale was widely used to evaluate the methodological quality of each RCT included for the meta-analysis [25]. This scale consisted of three evaluation elements: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points). One point would be allocated to each element if they were mentioned in the article, and another point would be given if the methods of randomization and/or blinding were appropriately described. The score of Jadad Scale varied from 0 to 5 points. If the Jadad score was ≥ 3, the study was thought to have high quality [26].
Statistical analysis
Standard Mean difference (SMD) with 95% confidence interval (CI) for continuous outcomes (FEV1 change, predicted FEV1 change, and peak expiratory flow change) and risk ratio (RR) with 95% CI for dichotomous outcomes (respiratory tract infection and treatment-related adverse events) were used to estimate the pooled effects. The random-effects model was used for all the meta-analyses. An I2 value greater than 50% indicated significant heterogeneity. Sensitivity analysis was performed to detect the influence of a single study on the overall estimate via omitting one study in turn when necessary. Publication bias was not assessed due to the limited number of included studies (n < 10). P < 0.05 in two-tailed tests was considered statistically significant. All statistical analyses were performed using Review Manager (RevMan Version 5.3. Copenhagen, Nordic Cochrane Centre, Cochrane Collaboration).
Results
Literature search, study characteristics and quality assessment
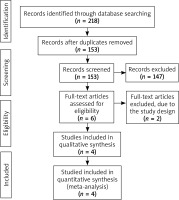
The flow chart for the selection process and detailed identification was presented in Figure 1. Two hundred and eighteen publications were identified through the initial search of databases. Ultimately, four RCTs were included in the meta-analysis [18–21].
The baseline characteristics of four eligible RCTs in the meta-analysis were summarized in Table I. The four studies were published between 2012 and 2014, and the total sample size was 451. Three studies involved OC 000459 200 mg twice daily [18, 19, 21], while the remaining study involved 25 mg once daily [20]. The treatment duration ranged from 8 days to 84 days.
Table 1
Characteristics of studies included
Among the four RCTs, three studies reported FEV1 change [18, 20, 21], and two studies reported predicted FEV1 change [18, 21], two studies reported peak expiratory flow change [18, 20], three studies reported respiratory tract infection and treatment-related adverse events [18–20]. Jadad scores of the four included studies varied from 3 to 5, and all four studies were considered to have high quality.
Primary outcomes: FEV1 change and predicted FEV1 change
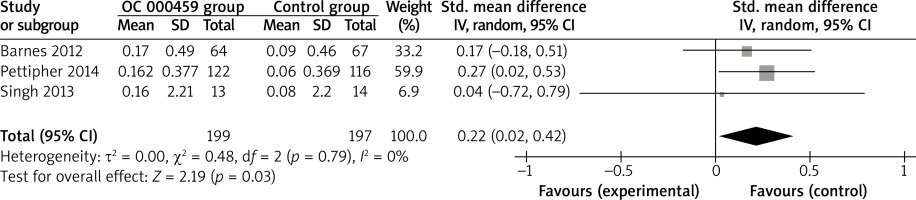
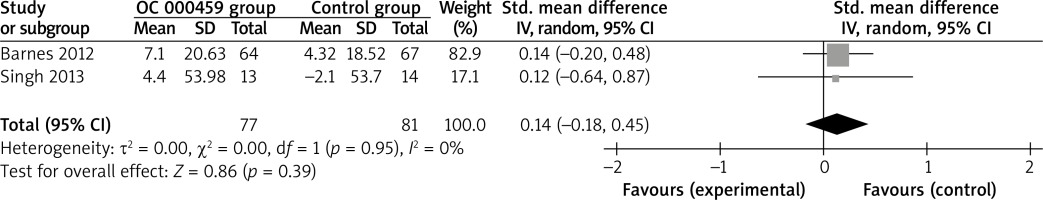
These two outcome data were analysed with the random-effects model. Compared to the control group for asthma patients, CRTH2 antagonist OC 000459 was associated with the increased FEV1 (SMD = 0.22; 95% CI: 0.02–0.42; p = 0.03) with no heterogeneity among the studies (I2 = 0%, heterogeneity p = 0.79) (Figure 2), but demonstrated no obvious effect on predicted FEV1 (SMD = 0.14; 95% CI: –0.18 to 0.45; p = 0.39) with no heterogeneity among the studies (I2 = 0%, heterogeneity p = 0.95) (Figure 3).
Sensitivity analysis
No heterogeneity was observed among the included studies for the primary outcomes. Thus, we did not perform sensitivity analysis by omitting one study in each turn to detect the source of heterogeneity.
Secondary outcomes
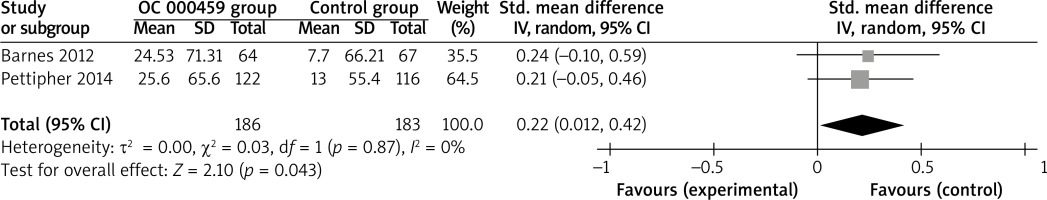
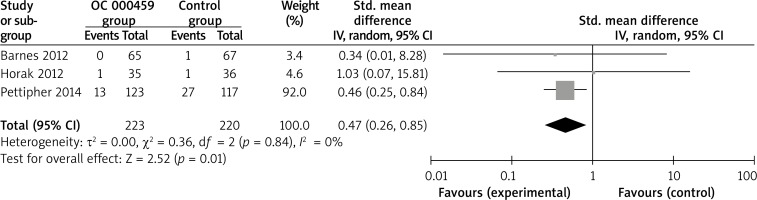
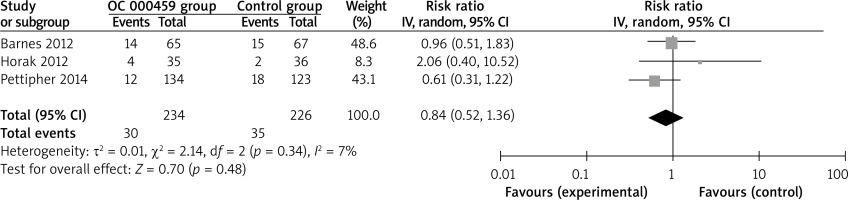
Compared with control intervention for asthma patients, CRTH2 antagonist OC 000459 can substantially increase the peak expiratory flow (SMD = 0.22; 95% CI: 0.01–0.42; p = 0.04; Figure 4) and reduce the respiratory tract infection (RR = 0.47; 95% CI: 0.26–0.85; p = 0.01; Figure 5). There was no statistical difference of treatment-related adverse events between two groups (RR = 0.84; 95% CI: 0.52–1.36; p = 0.48; Figure 6).
Discussion
PGD2 levels were raised in patients with asthma after the allergen challenge [27–29]. CRTH2 antagonist targeting this prostanoid could inhibit airway inflammation and mucus production in the mouse and guinea pig models of asthma [30, 31]. OC 000459, an N1-indole acetic acid derivative, was one selective antagonist of CRTH2. The improvement in pulmonary function was observed after 28-day OC 000459 treatment for asthma patients without inhaled corticosteroids [18].
Our meta-analysis suggested that CRTH2 antagonist OC 000459 had the capability to increase the FEV1 and peak expiratory flow change in asthma patients, but demonstrated no obvious effect on predicted FEV1 compared with placebo. In another study involving OC 000459 at three dose levels (25 mg once daily, 200 mg once daily or 100 mg twice daily) for 12 weeks, the efficacy of OC 000459 in improving lung function and asthma symptoms was observed in both atopic and non-atopic asthma subjects with varying degrees of blood eosinophilia. These three dose levels of OC 000459 were all effective, and 25 mg once daily of OC 000459 provided a higher increase in FEV1 and peak expiratory flow than the other two doses of OC000459. 25 mg (or less) administered once daily might be sufficient to provide maximal efficacy as evidenced by the high plasma concentrations of drug detected at this dose [20].
Blood eosinophil counts might have the utility in identifying responders to anti-IL-5, anti-IL-13 and anti-CRTH2 therapies, and were associated with disease severity and response to drug therapy. The geometric mean sputum eosinophil count was reduced from 2.1% to 0.7% (p = 0.03) after OC 000459 in asthma patients, but this effect was not significant in the placebo group [18]. Although there was no significant heterogeneity for the primary outcomes, different doses and treatment duration of CRTH2 antagonist OC 000459, and various severities of diseases might have some impact on the pooling results. In addition, CRTH2 antagonist OC 000459 resulted in the reduction in respiratory tract infection and no increase in the total treatment-related adverse events in our meta-analysis.
Several limitations should be taken into account. Firstly, our analysis was based on four RCTs only and two of them had a relatively small sample size (n < 100). Overestimation of the treatment effect was more likely in smaller trials compared with larger samples. Next, different doses and treatment duration of CRTH2 antagonist OC 000459, and various severities of diseases might have some influence on the pooling results. Finally, it was not feasible to perform the meta-analysis of some outcomes such as exacerbation and FVC based on current RCTs.