Summary
Transapical transcatheter aortic valve implantation in patients who are not suitable for a transfemoral approach gives a good hemodynamic result with significant clinical improvement. History of cerebrovascular event, impaired renal function, aortic valve area, increased NT-proBNP and right ventricular systolic pressure level may be associated with higher mortality at the 12-month follow-up.
Introduction
Aortic valve stenosis (AS) is the most common valvular disease, the frequency of which significantly increases with age. In patients older than 75 years, the incidence of 3.4% for severe AS represents a serious healthcare issue in Europe and in the United States [1–3]. For many years, a surgical valve replacement was the only effective method for AS treatment. Therefore, high risk surgery patients were very often left untreated and scheduled for pharmacological treatment [4]. Since the first endovascular implantation in 2002, transcatheter aortic valve implantation (TAVI) has become a valuable method for non-operable patients and an alternative to conventional surgical replacement [2]. During wider implementation of transcatheter heart valves, several approaches for TAVI were introduced. The transfemoral approach (TF) is the most common access route associated with the most favorable clinical outcomes [2, 5]. Patients undergoing TAVI constitute a very frail population with multiple comorbidities [6]. Computed tomography for access site evaluation often reveals diffuse severe atherosclerosis with massive calcifications and tortuosity in the iliofemoral region, which is a contraindication for femoral access. Transapical access (TA) represents one of the possible routes in patients who are not suitable for TF access. However, due to more aggressive intervention compared to the TF route and the need for general anesthesia as well as the higher risk profile of patients treated with TA, the apical approach is not often used nowadays and therefore many questions remain about the safety and clinical outcomes [7, 8].
Aim
The aim of our study was to assess early- and mid-term clinical outcomes after TA-TAVI in patients with severe AS.
Material and methods
A total of 61 consecutive high-risk elderly patients with severe symptomatic AS undergoing TA-TAVI from November 2008 to December 2019 were enrolled. Patient screening and selection were performed by a multidisciplinary Heart Team supported by clinical and imaging data. The clinical decision was based on logistic EuroSCORE I and the Society of Thoracic Surgeons (STS) Score [9]. Clinical assessment also included porcelain aorta, advanced liver cirrhosis, severe neurological impairment and frailty status. All procedures were performed by a cardiac surgeon and interventional cardiologist. All procedures were performed under general anesthesia and using Edwards Sapien, XT and Sapien 3 (Edwards Lifesciences, Irvine, USA) and ACURATE neo (Boston Scientific, Marlborough, USA).
After starting a TAVI program in our center, the number of all procedures annually was relatively low, starting from 30–40. Moreover, the TA route was a predominant access for TAVI, at least during the first year due to factors related to patient characteristics, including extremely high risk patients with multilevel atherosclerosis. Afterwards, TF access was gaining in frequency and we ended up with 120 procedures a year, 95% of which were performed via the TF approach. The number of TA procedures a year was highest between 2008 and 2014, reaching even 16 procedures a year, and since 2015 the number has dropped to 3–5 TA-TAVIs a year.
Baseline characteristics, procedural and outcomes data were collected. Endpoints of the study included all-cause mortality at discharge, 30 days and 12 months. Other endpoints were assessed according to the recommendations of the Valve Academic Consortium (VARC) [10]. The study protocol was approved by the institutional ethical board.
Statistical analysis
Categorical variables were presented as counts and percentages. Continuous variables were expressed as mean (standard deviation (SD)) or median (interquartile range (IQR)), where applicable. Normality was assessed via the Shapiro-Wilk test. Lifetime data were presented using the Kaplan-Meier estimator and analyzed using the Cox proportional hazards model. Simple models were created for all relevant variables (baseline, demographics). Due to the small number of observations, no multiple regression model was created. Two Kaplan-Meier curves were compared using a log-rank test. Hazard ratios (HR) with 95% confidence intervals (CI) were presented as a result of the Cox regression. Statistical analyses were performed in JMP 15.2.0 (SAS Institute Inc., Cary, NC, USA, 2020)
Results
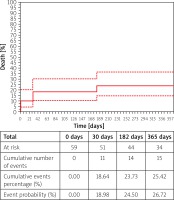
Sixty-one consecutive patients underwent TA-TAVI for native AS. Patients were elderly with median age of 80.0 years (76.00–84.0), 55.7% were male and 81.0% had symptoms of heart failure in III/IV class according to the New York Heart Association (NYHA). All patients were considered high risk according to median Logistic EuroSCORE I and STS scores 18.2% (11.6–27.7) and 4.8% (3.3–8.2), respectively. The median aortic valve area (AVA) was 0.70 cm2 (0.60–0.90) and the median value of the maximal/mean transaortic gradient was 82.0 mm Hg (59.5–93.5)/49.0 (36.0–61.0). Baseline clinical and echocardiographic characteristics are presented in Table I. The procedure success rate was 96.7%. All patients underwent balloon aortic valvuloplasty prior to valve deployment. All patients received only one prosthesis, so no valve-in-valve procedure was necessary. In 1 case a second valve was used due to problems with the delivery system. Conversion to open heart surgery was required in 2 cases, in 1 patient due to a severe paravalvular leak and in the second one due to a problem with apex cannulation. Procedural data and results are presented in Table II. All of the complications are presented in Table III. The remaining procedures were successful, with a good echocardiographic result. Median maximal and mean gradient values were 14.0 mm Hg (10.2–21.0) and 8.00 mm Hg (5.7–12.0), respectively. We found slightly lower median left ventricle ejection fraction at discharge than admission, 55.0 % (45.0–64.5) vs. 50.0% (43.5–60.0), p = 0.007. In most cases, we found at most only mild regurgitation of bioprosthesis, 90.2%. Periprocedural stroke occurred in 1 case, as did myocardial infarction. Although complications related to apex cannulation did not occur, the bleeding complication rate was 14.8%, while the need of transfusion of packed red blood cells occurred in 39.3%. Major bleeding complications were mostly associated with a second vascular access for pigtail insertion (n = 3). Two cases were associated with prolonged drainage from the index access site. Our registry showed that the need for pacemaker (PM) implantation, during index hospital stay after TAVI, occurred in 6.6%. Similarly, PM implantation was required in 6.6% of patients before the procedure, while on the waiting list. Six (9.8%) patients died during the index hospital stay. Three patients died due to bleeding complications related to gastrointestinal bleeding and retroperitoneal hematoma. In 2 cases septic shock and in one myocardial infarction were causes of 3 subsequent deaths. The 30-day mortality rate was 18.0%, and all-cause mortality at 12 months was 24.6% (Figure 1). Further analysis revealed factors which had a strong association with 12-month mortality. These were previous CVE (stroke or transient ischemic attack), glomerular filtration rate (GFR), AVA, right ventricular systolic pressure (RVSP) and serum level of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) (RR for CVE 3.17, 95% confidence interval (CI): 1.15–8.76: p = 0.026; RR for AVA per 0.1 cm2 1.28, 95% CI: 1.03–1.55: p = 0.024; RR for GFR per 1 ml/min 0.96: 95% CI: 0.94–0.99: p = 0.007; RR for NT-proBNP per 1000 pg/ml 1.07: 95% CI: 1.01–1.17: p = 0.033; RR for RVSP per 1 mm Hg 1.07: 95% CI: 1.02–1.16: p = 0.011 (Figure 2). Despite no statistical significance (p = 0.07), the NYHA class assessment at 30 days showed great clinical improvement; 93% of survivors were in class I or II. This beneficial effect was maintained after 6 months: 97.6% of patients in class I or II NYHA, p = 0.028.
Table I
Baseline characteristics of patients
[i] Data are presented as number and percentage. AVA – aortic valve area, AVMG – aortic valve mean gradient, AVPG – aortic valve peak gradient, BMI – body mass index, CABG – coronary artery bypass grafting, COPD – chronic obstructive pulmonary disease, CVE – cerebrovascular event – stroke or transient ischemic attack, EuroSCORE – European System for Cardiac Operative Risk Evaluation, GFR – glomerular filtration ratio, IQR – interquartile range, NT pro-BNP – N terminal pro B type natriuretic peptide, PCI – percutaneous coronary intervention, STS – Society of Thoracic Surgeons, RVSP – right ventricular systolic pressure.
Table II
Procedural data
Table III
Major periprocedural complications. Data are presented as number and percentage
| Complications | n (%) |
|---|---|
| Valve displacement | 0 |
| Second valve, bailout | 1 (1.6) |
| AVB requiring PM1 | 4 (6.6) |
| Cardiogenic shock | 4 (6.6) |
| Conversion to surgery | 2 (3.4) |
| Cardiac tamponade | 2 (3.6) |
| VARC 2 bleeding complications | 9 (14.8) |
| Major | 8 (88.9) |
| Minor | 1 (11.1) |
| Acute renal failure | 5 (8.2) |
| Blood transfusion | 24 (39.3) |
| Myocardial infarction | 1 (1.6) |
| Stroke | 1 (1.6) |
Discussion
Currently, TAVI gives the option for an effective treatment for patients who, according to the Heart Team decision, are not appropriate candidates for surgical aortic valve replacement [5]. While the transfemoral approach gives the best clinical outcomes, it cannot be used in everyone, particularly not in patients with severe iliofemoral atherosclerosis. Atherosclerosis is a systemic disease that included many vascular territories [11]. Peripheral artery disease often coexists with significant carotid artery stenosis [12]. In severe form, such as porcelain aorta, it makes it impossible to even use minimally invasive surgical techniques [13]. In those cases, TA is the second choice for transcatheter heart valve delivery, but due to its invasive character, bleeding and other complications remain a relevant concern [14]. After starting a TAVI program in our center, the TA route was a predominant access for TAVI, at least during the first year due to factors related to patient characteristics, including extremely high risk patients with multilevel atherosclerosis, and according to CT-scan assessment TF access was not possible in those cases. Our study confirmed that history of CVE, baseline impaired renal function as well as NT-proBNP, RVSP values and AVA may be associated with increased mortality.
In-hospital, 30-day and 12-month mortality rates were 9.8%, 18.0% and 24.6%, respectively. In hospital mortality, compared to estimated surgical risk according to logistic EuroSCORE I Scale 18.2%, was nearly two times lower. In a registry of all-comers with a similar recruitment timeframe, Bagienski et al. observed in-hospital, 30-day, 6-month and 12-month mortality of 6.9%, 10.9%, 15.8% and 17.8%, respectively [15]. The 30-day mortality in the study group was 18%. Some studies show that it can even exceed 20% [16]. On the other hand, in a study by Walter et al., the authors observed lower 30-day mortality with a survival rate of 91%, but with a similar death rate of 27% at 12 months of follow-up. In summary, currently available data showed relatively lower 30-day mortality rate at 12.2 % and only 3.8% in the PARTNER TA group; 12-month mortality was 32.2% and 29.0%, respectively [5, 14]. The 12-month all-cause mortality reached 24.6% but in the present study TA was the only possible method of invasive treatment in the group of patients with many comorbidities. Compared with the group of patients who received conservative treatment in the PARTNER I trial, 12-month mortality in the present study was two times lower (24.6% vs. 50.7%) [5].
In our study, bleeding, in the majority of cases life-threatening, was the most common complication, with a 14.8% frequency, yet complications associated with apex cannulation did not occur. These values are not greater than those available in other registries [17, 18]. Bleeding complication rate may be associated with the fact that the need of anticoagulation in patients with atrial fibrillation (AF) was 36.7%. TA may also be associated with new onset of AF [19]. Larger registries confirmed the impact of AF on bleeding complications [20]. Presence of AF is not only significant in terms of impact for bleeding complications but is strongly related with further mortality. In the FRANCE-2 registry all-cause mortality at 1 year in patients with pre-existing AF was 25.8% and was higher in those without AF [19].
The second most common complication was renal failure, with a 8.2% rate, which was probably associated with surgical trauma and the systemic inflammatory response, as a possible cause of injury [14, 21]. These results were similar to those previously described [18, 22, 23]. Another serious adverse event that may occur during TAVI is stroke [5].The frequency of periprocedural stroke and myocardial infarction in the study group was at the same level, 1.6%. This low value may be associated with the technical aspect of the procedure performed via the TA approach. The small distance between the sheath inserted through the apex and aortic valve makes positioning and controlled deployment much easier [2]. This fact may also have an impact on further procedure results.
The currently available registries made it possible to create a predictor model of unfavorable outcomes after TAVI. Factors with a significant influence are chronic oxygen therapy, advanced renal failure, atrial fibrillation, poor functional capacity, and decreased baseline cognitive function [24–26]. In our study we found that the history of CVE is strongly related to increased mortality. This fact may be related to TA’s more invasive character, which requires general anesthesia and could also cause some rehabilitation problems in the postprocedural period. Importantly, a history of CVE has been considered as a predictor of poor prognosis after cardiac surgery and included in the Society of Thoracic Surgeons (STS) risk score [24]. On the other hand, previous CVEs were not included in the EuroSCORE II risk score model. However, neither of these risk scores were designed for patients scheduled for TAVI [27].
Elevated RVSP is observed quite frequently in patients with AS [28]. Among patients undergoing TAVI, concomitant PH on echocardiography is found in 20–75% [29]. Also, in patients undergoing surgical aortic valve replacement for AS, baseline PH and its severity are associated with mortality, serious complications, and worse late survival [30]. Thus, patients with very elevated RVSP are often disqualified from surgical valve replacement due to concerns about high peri-operative morbidity and mortality or doubts about whether or not valve replacement will provide any clinical benefit. Data on the impact of PH on outcomes after TAVI are less consistent. In the study by Lindman et al. increased RVSP was associated with increased mortality, repeat hospitalizations, and strokes during the first year after TAVI [31]. Barbash et al. reported that the presence of RVSP > 50 mm Hg on echocardiography increased the mortality rate immediately after TAVI. In addition, patients with sPAP > 50 mm Hg had a prolonged hospitalization at the intensive care unit [32]. Also, another study confirmed a higher mortality rate at 12 months among patients with elevated RVSP [33]. In contrast, in the FRANCE-2 registry, the 30-day outcome did not differ among 2435 TAVI patients with sPAP < 40, 40–60, and ≥ 60 mm Hg as assessed by echocardiography [34].
Previous studies have confirmed that elevated levels of NT-proBNP may be associated with worse outcomes [35, 36]. Koskinas et al. reported that an increased value of NT-pro BNP is associated with a higher risk of all-cause death and cardiovascular death at 2 years and more frequent VARC-2 complications. On the other hand, Ben-Dor et al. did not confirm the significant impact of BNP values on further mortality [37]. Moreover, RVSP and NT-proBNP are not included in risk scores such as the logistic EuroSCORE and STS.
Our study had a relatively small sample size. Data were analyzed retrospectively as a registry from a single center.
Conclusions
Transapical TAVI in patients who are not feasible for a transfemoral approach gives a good hemodynamic result with significant clinical improvement. History of CVE, impaired renal function, AVA, increased NT-proBNP and RVSP level may be associated with higher mortality at the 12 months follow-up.