Introduction
Hepatocellular carcinoma (HCC) is a highly lethal malignancy, with a 5-year survival rate of 5-15% [1, 2]. The poor prognosis of HCC is related to the underlying liver disease and due to the fact that around 50% ofpatients are diagnosed at an advanced stage, when no curative alternatives are feasible [3]. In these cases, systemic therapies aim to improve survival by delaying disease progression and clinical deterioration.
The upfront standard treatment for patients with extrahepatic spread is based on drugs tested in phase III randomized trials. The combination of atezolizumab (an anti-PD1 antibody) and bevacizumab (an anti-VEGF antibody) has been the standard of care since 2020 [4], while the multikinase inhibitors sorafenib and lenvatinib are reasonable options in patients ineligible for immunotherapy or in regions where the combination is not available [5].
Hepatocellular carcinoma often metastasizes through hematogenous spread, lymphatic dissemination and direct invasion. The usual sites of metastasis are lungs, bones, lymph nodes and adrenal glands. Nevertheless, HCC can rarely present with peritoneal metastasis (PM) in 2-15% of cases [6, 7].
Particular risk factors have been suggested to be associated with PM, such as tumor rupture or needle tract seeding following biopsies or percutaneous ablation procedures. The prognostic impact of peritoneal dissemination is not well established [8, 9]. Data suggest that liver function and intrahepatic disease control are determinants of outcomes in patients with PM [10]. Therefore, the optimal approach for this subset of patients should be based on a multidisciplinary approach and focused on maintaining a preserved hepatic function, rational use of systemic treatments and surgical approaches in selected cases. The aim of this study is to report clinical-pathological characteristics and outcomes of patients with HCC and peritoneal spread treated through multidisciplinary decisions.
Material and methods
Study design and participants
We evaluated a retrospective cohort of patients with advanced HCC treated between October 2009 and January 2018 at our institution. All patients included in the analysis met diagnostic criteria for HCC based on radiological and/or histological findings [11]. Clinical characteristics related to underlying liver disease and information about treatments and clinical outcomes were collected from medical records.
Patients were excluded in case of: 1) diagnosis of fibrolamellar HCC or mixed hepatocellular tumors, 2) insufficient data in medical records, or 3) loss of follow-up that impair data analysis.
The site of metastatic spread was determined according to the baseline imaging routinely performed: abdominal and thoracic computed tomography, abdominal magnetic resonance imaging and bone scintigraphy.
Patients were further divided into two groups: 1) those with PM and 2) those without PM. Among patients with PM, we identified those patients who have been submitted to peritonectomy. Comparisons of features and outcomes were performed between patients with PM vs. no PM and between patients with PM who were treated with systemic treatment or upfront peritonectomy. The study was approved by the institutional ethics committee (protocol number 3.807.496).
Treatment
According to the institutional protocol, the standard management of advanced stage disease is systemic therapy. Nevertheless, weekly multidisciplinary boards (composed of surgeons, clinical oncologists, hepatologists, radiologists and pathologists) discuss individual cases for which personalized management can be offered. Patients with low burden disease, preserved liver function, no significant comorbidities and features that suggest an indolent biological behavior (late recurrence, absence of symptoms and low α-fetoprotein serum levels) are considered for local modalities and/or surgery for metastatic sites. Before 2008, the use of systemic treatment was limited due to the lack of approved drugs. In 2008, sorafenib was approved based on the results of the SHARP trial [12].
During the study period (2009-2018), the recommended systemic treatment was sorafenib at a dose of 400 mg twice daily until disease progression, unacceptable adverse events or death. The use of reduced doses was permitted from the beginning and during the course of treatment (depending on tolerability and side effects) at the discretion of treating physicians. The follow-up consisted of regular clinical visits, laboratory tests every three to four weeks and assessment of radiological response (computed tomography or magnetic resonance) every eight to twelve weeks.
In patients with PM for whom peritonectomy was indicated, the procedure started with diagnostic laparoscopy to assess feasibility and disease burden. If a decision was made to proceed with surgery, a midline laparotomy was performed and the peritoneal carcinomatosis index (PCI) was registered. PCI is a widely used system that quantitatively describes the distribution and size of the peritoneal spread throughout 13 abdominal regions: the size of the largest implant is scored for each region and the sum of each region’s score results in the PCI score, with a range of 1-39 [13]. Due to the retrospective design, there was no predefined PCI cutoff, although a PCI higher than 16 was considered unsuitable for peritonectomy by most of the board members. Tumor debulking was performed as dictated by the disease burden and distribution, which included resection of intra-abdominal organs, peritoneal nodules, affected peritoneal surfaces and suspected lymph nodes. Post-surgery surveillance consisted of physical examination, imaging tests and α-fetoprotein (AFP) measurement every 3-4 months.
Statistical analysis
Continuous variables were expressed as mean, median, ranges or interquartile intervals. Categorical variables were expressed as frequency. Categorical variables were compared using the χ2 test or Fisher’s exact test where appropriate. Overall survival (OS) was estimated using the Kaplan-Meier method and curves were compared by log-rank test. Univariate and multivariate analyses using the Cox proportional hazards model were performed to evaluate prognostic factors. Variables were included in the multivariate analyses if they presented a p value < 0.05 in the univariate analysis and were not associated with each other. Data were evaluated using STATA software version 15.0.
Results
Patients’ characteristics
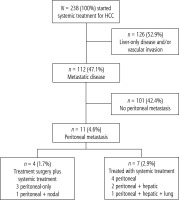
Between October 2009 and January 2018, 238 patients with advanced HCC were treated and included in the present analysis. From the total cohort, median age was 61.8 years (range: 18.9-84.9), 189 (79.4%) were male and the predominant underlying liver disease was hepatitis C (n = 112, 47.1%), followed by hepatitis B (n = 46, 19.3%) and alcohol-related liver disease (n = 36, 15.1%). According to the Barcelona Clinic Liver Cancer group (BCLC) staging, 151 (63.4) were stage C while 41 (17.2%) were stage B. Most of the patients had Child-Pugh A (n = 205, 86.1%) and Eastern Cooperative Oncology group (ECOG) performance status of 0 (n = 171, 71.8%). Extrahepatic disease was present in 112 (47.1%) of the patients at sorafenib initiation and the frequent metastatic sites were lungs (n = 48, 42.8%), lymph nodes (n = 21, 18.8%) bones (n = 20, 17.9%), adrenal gland (n = 16, 14.3%) and peritoneal (n = 11, 9.8%). Forty-one (17.2%) patients did not receive any curative modalities previously, while 197 (82.8%) had received curative modalities before systemic treatment (Table 1). Figure 1 shows the flow chart of the study.
Table 1
Baseline patient demographics and disease characteristics
Patients with peritoneal metastasis
Eleven patients had PM, representing 9.8% of the patients with extrahepatic disease and 4.6% of the total cohort. The peritoneum was the only site of disease in 7 patients, while 4 other patients had concomitant sites (hepatic [n = 3] and nodal [n = 1]). Median age of the cohort with PM was 46.5 years (range: 43.8-51.3), 5 (45.4%) patients had hepatitis C, 4 (36.4%) patients had hepatitis B and 2 (18.2%) patients had no concomitant liver disease.
Two patients had de novo HCC with peritoneal spread, while 9 other patients had recurrent PM after having received locoregional treatment: 2 patients had been submitted to liver transplantation, 1 patient had received transarterial chemoembolization, 5 patients had been treated with hepatic resection and 1 patient received percutaneous ablation combined with transarterial chemoembolization. Ten out of 11 patients had well-preserved liver function, while 1 (9.1%) patient had Child-Pugh B.
There were no significant differences between patients with and without PM regarding clinical and pathological characteristics, except that those patients with PM were significantly younger than those with no PM (p = 0.035). Table 1 shows the baseline demographic characteristics of the cohort and Table 2 details the 11 patients with PM.
Table 2
Baseline features and treatment for patients with peritoneal metastasis
Patients submitted to peritonectomy
Four patients with PM were submitted to peritonectomy as the upfront treatment for metastatic disease (Fig. 1). These cases were individually discussed in multidisciplinary tumor boards and clinical characteristics, tumor burden and feasibility of surgery were considered for decision making. Table 2 shows the main baseline features of this subgroup. All 4 patients were Child-Pugh A, had no macrovascular invasion, no ascites and a median alpha-fetoprotein serum level of 53 ng/ml (range: 11.9-188). Three patients had peritoneal disease as the only site of disease and one patient had both PM and lymph node involvement. All patients who were submitted to surgery had a R0 resection. There was no surgery-related mortality.
All 4 patients had recurrences, with a median time from surgery to recurrence of 30.25 months (interquartile range [IQR]: 13.53-46.92): 3 of them with PM (2 diffuse peritoneal spread, 1 with concomitant hepatic recurrence) and 1 with lung recurrence.
Clinical outcomes
Considering the entire cohort of patients with advanced HCC who started systemic treatment (n = 238), the median OS was 8.1 months (95% confidence interval [CI]: 6.76-10.0), with a median follow-up of 7.9 months (IQR: 6.8-8.8]. The median OS of the subgroup that did not present PM (n = 227) was 8.1 months (95% CI: 6.73-10.0).
Regarding patients with PM (n = 11), the median OS was 17.9 months (95% CI: 2.77-not-reached [NR]). Although numerically superior, there was no statistically significant difference in OS comparing the group with and without PM (log-rank p = 0.51).
Considering those patients who were not candidates for peritonectomy, the median OS was 11.77 (95% CI: 1.47-19.81) with a median follow-up of 14.83 months (IQR: 2.77-26.30). Those patients who were treated with peritonectomy upfront followed by sorafenib at recurrence had a median OS of 60 months (95% CI: 16.7-NR). This was statistically superior compared to the PM group not candidates for surgery treated only with systemic treatment (log-rank p = 0.028; Cox regression adjusted by Child-Pugh and ECOG performance status: hazard ratio [HR]: 0.12, 95% CI: 0.014-0.891) (Fig. 2). In the last follow-up, 2 patients in the PM group were still alive and under systemic treatment; both had been submitted to peritonectomy (Table 3).
Fig. 2
Kaplan Meier curves. A) Patients with peritoneal metastasis submitted to surgery plus systemic treatment vs. patients treated with systemic treatment; B) Patients with peritoneal metastasis vs. patients with no peritoneal metastasis
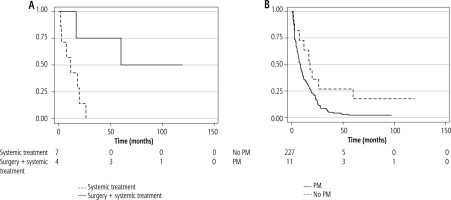
Table 3
Outcomes of patients according to the presence of peritoneal metastasis and the treatment received
Discussion
This article describes a large cohort of patients with advanced HCC and detailed clinical characteristics and outcomes of patients with PM. A subset of patients with PM was treated with surgery and showed long-term recurrence-free survival. These patients presented an encouraging survival time of 60 months, which highlights the need to individualize treatment strategies for HCC patients with PM.
According to international guidelines, the management of patients with extrahepatic spread is based on systemic treatment [14, 15]. In the past years, a variety of novel drugs were incorporated and the prognosis of advanced HCC improved significantly [4, 16]. Prospective studies in advanced HCC reveal that around 10-30% of patients may present deep and durable responses, which opens the opportunity for discussing the combination of systemic treatment and local modalities, such as resection of metastatic disease [17]. Moreover, it is clear that biological behavior is a crucial determinant of the benefit from a more aggressive strategy in advanced HCC, such as surgical treatment of metastasis.
Peritoneal spread is reported to occur in up to 2-18% in autopsy series [18, 19]. The mechanisms that drive PM have not been well established, although it is suggested that malignant cells may disperse into the abdominal cavity during HCC spontaneous rupture, percutaneous biopsy and percutaneous ablation treatments [20, 21]. These risk factors were observed in a few patients from our cohort, but a clear association was not possible due to the small sample size. Other unrecognized mechanisms may also play a role in PM genesis, such as the differentiation grade and the presence of macrovascular invasion, which deserves further investigation [18].
Some groups have published small series and case reports suggesting that, in addition to systemic treatment, surgical treatment of PM may provide favorable outcomes in selected HCC patients [18-21]. Lin et al. reported the results of cytoreductive surgery in a selected group of patients with predominant peritoneal disease and a low disease burden. Survival was shown to be superior in the group treated with surgery compared to systemic treatment [19]. Tabrizian et al. also reported long-term survival in a subgroup of 14 patients submitted to cytoreductive surgery, with low rates of perioperative complications [22]. Some of these studies also reported the feasibility of adding hyperthermic intraperitoneal chemotherapy (HIPEC) in diverse malignancies [23], but the use of HIPEC requires further evaluation in prospective studies to determine safety, once HCC patients have limited tolerance of cytotoxic chemotherapy due to coexisting liver disease.
In our series, 4 patients were treated with upfront surgery, which provided a long recurrencefree survival time of around 30 months. At recurrence, patients received systemic treatment, with a similar response compared to those who received systemic treatment as the first treatment for PM or other sites of disease. A close follow-up after surgery ensured that all recurrences were detected before clinical deterioration and liver impairment, so that patients had a favorable performance status to receive systemic treatment.
A key aspect in our study is that these 4 patients had favorable baseline characteristics that encouraged a more aggressive approach. All 4 patients had no severe comorbidities, preserved liver function, no ascites, no concomitant hepatic disease, no major vascular invasion, low serum α-fetoprotein levels and a PCI < 10. Additionally, this subgroup was younger compared to the total cohort.
Limitations of the present study are the small sample size and its retrospective nature. Nevertheless, multidisciplinary tumor boards involving all the specialties dedicated to liver cancer should dictate the best approach for HCC patients with PM who present other factors of better prognosis, such as Child-Pugh A, low tumor burden and stable hepatic disease.
Conclusions
Peritoneal metastases from HCC are rare and include a heterogeneous range of clinical presentation, biological behavior and outcomes. The prognosis varies according to clinical characteristics and response to treatment. A multidisciplinary approach is mandatory for selecting patients who derive benefit from surgery for PM and a close follow-up allows the early detection of recurrence and proper indication for systemic treatment.