Introduction
Chronic hepatitis C virus (HCV) infection progresses slowly and leads to cirrhosis in 20-30 years. After the development of cirrhosis, the risk of progression of liver damage to hepatic decompensation is approximately 3-6%/year [1]. Patients with decompensated cirrhosis, Child-Turcotte-Pugh (CTP) class B or C, have a high risk of developing portal hypertension-related complications such as ascites, jaundice, variceal bleeding, or hepatic encephalopathy in due course of time. However, oral direct-acting antivirals (DAAs) have markedly revolutionized the management of patients with chronic HCV infection. In patients with compensated cirrhosis, sustained virological response (SVR) has been more than 90% and around 80% in cases of decompensated cirrhosis [2-5]. Successful HCV treatment has been shown to lower the risk of hepatic failure, hepatocellular carcinoma (HCC), liver-related deaths, and all-cause mortality. Approximately one-quarter of patients with compensated cirrhosis no longer have liver stiffness in the cirrhotic range within 1 year of SVR [6]. However, in cases of decompensated cirrhosis, it is presently not clear whether viral clearance results in a lasting improvement of signs of liver decompensation. Although the definitive treatment for patients with decompensated cirrhosis is liver transplantation (LT), recent studies have shown meaningful biochemical and clinical improvement in patients with HCV-related decompensated cirrhosis on DAA therapy. Patients listed for LT due to decompensated cirrhosis had improvement in their liver function to an extent that some of them were inactivated from the waiting list and eventually delisted [7,8].
In the past, it has been observed that when LT candidates with decompensated cirrhosis due to hepatitis B virus (HBV) related liver disease were treated with nucleo(s/t)ide analogues (NUC), almost one third of these patients were eventually delisted while on NUC therapy and their clinical improvement could be maintained for up to 5 years [9]. Patients with untreated hepatitis C or failed treatment who underwent LT can have a significantly accelerated disease course compared to a non-LT setting. Between 10% and 30% developed cirrhosis within 5 years from LT and 40% presented signs of liver decompensation within 1 year from the diagnosis of recurrent cirrhosis [10-12]. Due to the effectiveness of DAA therapy the prevalence of HCV infection is expected to decline but hepatocellular carcinoma and liver-related deaths are expected to continue rising worldwide until 2030 [13].
Few studies have looked for factors that are associated with response to DAA therapy in patients with HCV-related liver disease. Ahmed et al. found that factors responsible for poor response to DAAs were older age, cirrhosis, especially CTP class B, and low platelet count found in an Egyptian study using sofosbuvir and daclatasvir for genotype 4 [14]. In another study reduced response rates occurred more frequently in treatment-experienced patients, those with advanced cirrhosis, HCV genotypes 3 or 1a infections, elevated serum HCV-RNA, poor drug adherence or premature drug discontinuation [15]. Recently a new score (BE3A) has been devised using the sum of five factors, namely body mass index (BMI) < 25 kg/m2 , absence of encephalopathy, absence of ascites, alanine transaminase (ALT) > 60 IU/l, and albumin > 3.5 g/dl, which were identified to be associated with clinical recovery from decompensated cirrhosis after DAA treatment [16]. Patients with a BE3A score of 4 have a 75% chance of recovering to CTP class A after DAA treatment.
There is scanty literature regarding predictive factors of response in cases of decompensated HCV-related cirrhosis. Hence, in this study, we looked for factors which are responsible for the response to DAA therapy and achieving a clinically meaningful treatment benefit which was defined as reduction to CTP class A (i.e. down-staging from CTP class B to A or C to A) sustained until the end of follow-up. We also devised a new model and compared it with the validated BE3A score.
Material and methods
Patients
Patients treated from January 2016 to August 2018 were prospectively included. Written consent was taken from all patients. The study was approved by the Institute’s Ethics Committee. All patients who were age 18 years or older and having chronic HCV infection of genotype 1-6 and decompensated cirrhosis (CTP B or C) were included. Abdominal ultrasonography and biochemical tests including liver function tests and serum α-fetoprotein (AFP) were done in all patients. A triple-phase computed tomography (CT) scan of the abdomen was considered for patients with raised serum AFP. Patients diagnosed to have HCC were excluded from the study. Patients having co-infection with human immunodeficiency virus (HIV), hepatitis B virus (HBV) and previous exposure to DAAs were also excluded. Moreover, patients with any of the following baseline laboratory parameters were also excluded: platelets < 30,000/mm3, ALT, aspartate aminotransferase (AST), or alkaline phosphatase (ALP) more than 10 times the upper limit of normal or estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2 .
We excluded 13 out of 75 patients with decompensated cirrhosis because they had: HBV co-infection (4 patients), HCC (5 patients), previous exposure to DAA (2 patients) and death unrelated to cirrhosis (2 patients). The remaining 62 patients were included in our study. Written informed consent was taken from each patient.
Clinical, anthropometric and laboratory evaluation
Independent variables based on clinical relevance to cirrhosis and liver decompensation were noted, which included weight, height, BMI, complete blood count (CBC), liver function test (LFT) including total bilirubin, ALT, AST, albumin, international normalized ratio (INR), serum creatinine and eGFR. Quantitative HCV-RNA using the TaqMan Real-Time PCR technique and HCV genotype were analyzed for each patient. Clinical variables such as presence of ascites, encephalopathy and any evidence of gastrointestinal bleeding were also noted. All variables were categorized to facilitate clinical interpretation. Binary variables were adopted for presence or absence of ascites and encephalopathy. Conventional cut-off points for the Indian population were used for BMI. Body weight was adjusted based on the severity of ascites and the presence of pedal swelling before measuring BMI. A percentage of body weight was subtracted from total body weight based on the severity of ascites (mild 5%, moderate 10%, severe 15%), with an additional 5% subtracted if bilateral pedal edema was present.
The BE3A score was calculated by the sum of its 5 components; each had 1 point when the criterion was met.
The Model for End-Stage Liver Disease (MELD) score and CTP score were calculated for each patient using appropriate variables.
DAA therapy was considered for each patient. Sofosbuvir 400 mg along with daclatasvir 60 mg or velpatasvir 100 mg with or without ribavirin was considered in patients with HCV genotype 2 or 3 infection, whereas sofosbuvir 400 mg along with ledipasvir 90 mg with or without ribavirin was considered for HCV infection with genotype 1, 4, 5 or 6 infection [17]. Daclatasvir was used initially until July 2017 in the study period when velpatasvir was not available in India. Ribavirin was used according to body weight (1000 mg/day if weight < 75 kg, 1200 mg/day if weight > 75 kg). Ribavirin was started at a low dose for each patient (600 mg) and gradually increased at a dose of 200 mg/week to the target dose if tolerated based on hemoglobin values. In the course of treatment, if hemoglobin dropped below 10 g/dl, the ribavirin dose was reduced by 200 mg and was stopped in patients whose hemoglobin dropped below 8.5 g/dl. Ribavirin was not used in those whose baseline hemoglobin was < 10 g/dl [18].
Treatment was continued for 12 weeks in those in whom ribavirin was used and for 24 weeks for the rest of the patients.
Routine investigations including CBC, LFT, creatinine and INR along with clinical assessment for the presence of ascites and encephalopathy were done at each patient’s follow-up. Every patient was followed up after two weeks of starting therapy and then every 4 weeks. Patients in the ribavirin group were assessed with CBC weekly for 4 weeks until the target dose was achieved, then they were monitored every two weeks to look for hematological side-effects.
Quantitative HCV-RNA was analyzed at 4 weeks to look for a rapid virologic response (RVR) then at end of treatment, and finally at 12 weeks after treatment to look for SVR. CTP and MELD scores were calculated at every follow-up visit.
All included patients were followed up for 36 weeks after treatment. Any decompensating events while on treatment or in the post-treatment period were recorded for each patient.
Statistical analysis
Descriptive and inferential statistical analysis was carried out in the present study. Results on continuous measurements are presented as mean ± SD (min-max) and results on categorical measurements are presented as numbers (%). Student’s t-test (two-tailed, independent) was used to determine the significance of study parameters on a continuous scale between two groups (Intergroup analysis) on metric parameters. Leven’s test for homogeneity of variance was performed to assess the homogeneity of variance.
Chi-square/Fisher’s exact test was used to find the significance of study parameters on a categorical scale between two or more groups, the non-parametric setting for qualitative data analysis. Fisher’s exact test was used for a small sample size.
Univariate analysis was performed to estimate odds ratio (OR) for age, sex, components of CTP score, albumin, ALT, eGFR, as well as its components. Multivariate analysis was also done for the same variables to estimate OR. The results were used to generate a model for predicting response to DAA therapy in decompensated HCV-related cirrhosis. The newly devised model was then compared with the validated BE3A score to evaluate its performance in a real-life scenario.
Statistical software: SPSS 22.0, and R environment ver.3.2.2 were used for the analysis of data and Microsoft Word and Excel were used to generate graphs, tables, etc.
Results
Demographic profile
Sixty-two patients with decompensated cirrhosis due to HCV were included. The treatment options available, along with the side-effect profile of existing drugs, were explained to all patients.
Patient characteristics
All were treatment-naïve cases of decompensated cirrhosis due to HCV infection. The median age of our study population was 52 years (42-80), with males constituting 36 (58.1%) of all. Out of 62 patients, 55 (88.7%) patients belonged to CTP class B and the remaining 7 (11.3%) had CTP class C. Genotype 3 was the most common genotype causing HCV infection in our study population, with 50 cases (80.7%), followed by genotype 1 in 11 (17.7%) and genotype 4 in 1 (1.6%). The median HCV-RNA count in our patient population was 2,53,000 (2,300-3,38,60,000) IU/ml.
Demographic and baseline characteristics are shown in Table 1.
Table 1
Demographic and baseline characteristics of patients
Virological outcomes
Virological clearance, defined as HCV-RNA < lower limit of quantification with a detection threshold of 15 IU, was observed in 62 (100%) patients after 4 weeks of therapy (RVR = 100%) and in 60 (96.7%) patients after 12 weeks of treatment. Two patients failed to clear HCV RNA after 12 weeks of treatment. Both of them were on sofosbuvir and velpatasvir and treatment was continued for 24 weeks. Ribavirin could not be used in either because of anemia.
The number of patients receiving sofosbuvir combination therapy with daclatasvir, ledipasvir or velpatasvir was 27 (43.5%), 11 (17.8%) and 24 (38.7%) respectively. Ribavirin was used in 24 (38.7%) patients with no contraindication to it along with sofosbuvir combination therapy.
The total number of responders, i.e. attainment of CTP class A state, was 34 (54.8%) and 28 (45.2%) were non-responders.
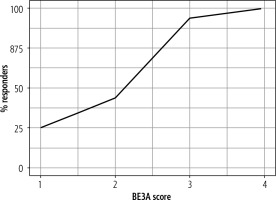
The total number of responders was higher in patients with a higher BE3A score (Table 1). The number of responders was 3 (25%), 14 (43.75%), 15 (93.75%) and 2 (100%) with a BE3A score of 1, 2, 3 and 4 respectively (Fig. 1).
Impact of DAA on biochemical parameters
The pre-treatment and post-treatment values of various biochemical parameters are tabulated in Table 2.
Table 2
Biochemical parameters in pre-treatment and post-treatment period
Clinical profile of patients
Clinical outcome
Death: Two patients (3.2%) died during the study period. One death was recorded in the 2nd week of treatment in a patient who had advanced decompensated liver disease. The other one was non-compliant to therapy with decompensation and acute kidney injury and died during the 4th week of therapy.
Decompensating events
Out of 60 patients who attained SVR 12, 36 (58%) patients did not have any decompensating events in the follow-up period, whereas 24 (38.7%) patients were noted to have presented with one or more decompensating events in the follow-up period.
Two patients who failed to achieve SVR12 had one or more decompensating events in the follow-up period.
Impact of DAA on clinical parameters
Before DAA therapy mild to moderate ascites was seen in 49 (79%) patients and severe ascites in 13 (31%) patients. After treatment 45 (72.6%) patients had complete resolution of ascites, 16 (25.8%) patients had persistent mild to moderate ascites and one (1.6%) patient had severe ascites.
In our study population, 3 (4.8%) patients had a history of encephalopathy (West Haven grade 1 and 2) before treatment. No patients had a history of West Haven grade 3 or 4 encephalopathy. After treatment 1 (1.6%) had a history of encephalopathy for which hospitalization was required.
Impact on CTP score
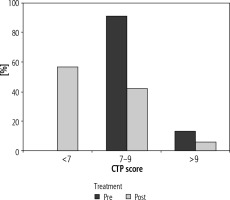
In the pre-treatment period, the numbers of patients in CTP class B and C were 55 (88.7%) and 7 (11.3%) respectively. After DAA therapy there was a marked improvement in CTP score. The number of patients in CTP class A, B, and C were found to be 34 (54.8%), 25 (40.3%) and 3 (4.8%) respectively (Fig. 2).
Impact on MELD score
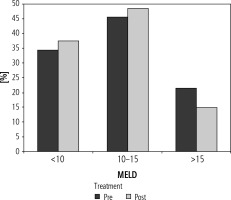
The number of patients with a MELD score < 10, 10-15 and > 15 in the pre-treatment period were 21 (33.9%), 28 (45.1%) and 13 (21%) respectively. In the post-treatment period, the number of patients with a MELD score < 10 increased to 23 (37.1%), and that in 10-15 and > 15 groups was 26 (48.4%) and 9 (14.5%) respectively (Fig. 3).
Fig. 3
Bar diagram showing patients with baseline MELD score <10, 10-15 and >15 in pre- and post-treatment period
Delta MELD was calculated using the difference in MELD values at baseline and post-treatment period. The mean value of delta MELD in treatment responders, i.e. CTP class A patients, and non-responders was 1.35 ±1.81 and 0.46 ±2.87 respectively and was not statistically significantly different (p = 0.214). Similarly, delta albumin was calculated from the difference in serum albumin values in the pre- and post-treatment periods. Mean value in responders and non-responders was 0.34 ±0.39 g/dl and 0.23 ±0.43 g/dl respectively (p = 0.147).
On univariate analysis, serum bilirubin (OR = 0.41, 95% CI: 0.20-0.75), albumin (OR = 4.84, 95% CI: 1.43-20.15) and eGFR (OR = 1.03, 95% CI: 1.0-1.06) and on multivariate analysis only serum bilirubin (OR = 0.28, 95% CI: 0.1-0.64) were found to be predictors of attainment of CTP class A after DAA therapy in decompensated HCV cirrhosis patients (Table 3).
Table 3
Summary table of logistic regression of responder against predictors
Deriving our new model
A new model was devised with the parameters age, gender, platelet, serum bilirubin, ALT, albumin, INR, eGFR and clinical variables – the presence of ascites and encephalopathy – for predicting response to DAA therapy and attainment of CTP class A after treatment.
We fitted a logistic model (estimated using ML) to predict responders with age, gender, serum bilirubin, albumin, INR, ALT, eGFR and presence of ascites and encephalopathy. Standardized parameters were obtained by fitting the model on a standardized version of the dataset. Effect sizes were labeled following Chen’s (2010) recommendations.
The probability was calculated from the odds ratio using the following equation:
Probability = Odds / 1 + Odds,
where the odds for achieving CTP A status was expressed as:
Odds [Improvement] = e – 0.018 × [Age] – 0.6345 × [Gender] – 1.2786 × [Bilirubin] – 0.0891 × [Ascites] + 1.9126 × [Albumin] – 0.8392 × [INR] + 0.0351 × [ALT] – 1.9133 × [Encephalopathy] + 0.036 × [eGFR] – 0.0018 × [Platelet count] – 2.7895.
In which 1 was used for the presence of encephalopathy or ascites, and 0 for its absence respectively, for age < 65 years 0 and > 65 years 1 were used, for gender 0 and 1 were used for female and male respectively, with bilirubin in unit of mg/dl, albumin in unit of g/dl, ALT in unit of IU/l, eGFR in ml/min/1.73 m2 and platelet count in 106/ml.
Metrics of logistic regression using our model revealed an Akaike information criterion (AIC) and Bayesian information criterion (BIC) of 79.9 and 103 respectively.
Using the BE3A score as a predictor of treatment response, metrics of logistic regression revealed an AIC and BIC of 71.4 and 75.65 respectively.
Confusion matrix of our model and BE3A score
Our new model had sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 67.86%, 79.41%, 73.08%, 75%, and 73.63% respectively in predicting response to therapy. Similarly, with BE3A score sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of predicting response to DAAs were 32.14%, 91.18%, 75%, 62%, 61.66% respectively.
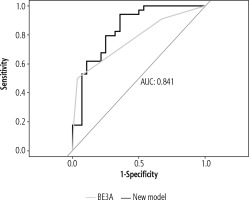
However, comparison of our model with BE3A score using ROC analysis revealed better results with our composite model, with an AUC value of 0.841 and 0.771 respectively, although DeLong’s test for two correlated ROC curves revealed a statistically non-significant result (p = 0.29) (Fig. 4).
Discussion
We have analyzed all 62 patients with advanced cirrhosis who underwent antiviral therapy with sofosbuvir-based DAA therapy at our center. Assessment of virologic efficacy and frequency of clinical events reflecting hepatic decompensation were studied and predictive parameters for patients at risk for decompensation were looked for. The overall SVR rate in our patient cohort was 96.8%. Thirty-four (54.83%) patients attained the primary end-point of our study, i.e. attainment and persistence of a compensated state in the post-treatment follow-up period of 36 weeks. The rate of hepatic decompensation defined as the onset of ascites, hepatic encephalopathy, upper gastrointestinal bleeding or new-onset jaundice in the follow-up period was 45.2%.
At short-term follow-up (12 weeks), 47-68% of patients with decompensated HCV cirrhosis who achieved SVR did not require liver transplantation and had CTP score improvement. However, 11-24% had worsening of CTP scores [5,19,20].
In our study population, baseline serum bilirubin, ALT, MELD score and CTP class were significantly associated with response to DAA treatment. Responders, i.e. patients who had persistent CTP class A following treatment even in their follow-up period, had baseline ALT > 1.5 times the upper limit of normal compared to non-responders. A similar finding was reported by El-Sherif et al. [16].
In patients with a MELD score of < 15, 33 out of 49 (67.3%) successfully met the primary end-point of our study, whereas 16 (32.7%) patients failed to do so. However, only 1 in 13 (7.6%) patients with a MELD score > 15 attained a state of compensated liver disease after DAA therapy. In CTP class B, 34/55 (61.8%) succeeded in attaining CTP class A status after treatment. None of the patients with a baseline CTP score > 9, i.e. CTP class C, could attain a persistent compensated state, i.e. CTP A, despite DAA therapy in the follow-up period of 36 weeks.
Among patients with CTP class B cirrhosis or a low (< 15-18) MELD score, 35-61% had improved MELD scores, whereas 22-33% had worsened MELD scores. Among patients with CTP class C cirrhosis or a high (> 15-18) MELD score, 81-87% had improved MELD scores, whereas 13-33% had worsened MELD scores [5,19,20].
The presence of SVR12 was not significantly associated with response to DAA treatment (p = 0.2). Only 2 patients in our study group who failed to maintain persistent CTP class A after treatment did not achieve SVR12. One patient was hospitalized twice during follow-up with tense ascites requiring large-volume paracentesis. The second patient also had a history of ascites twice in the follow-up period, requiring up-titration of diuretics.
On univariate analysis, factors that were significantly associated with improvement in liver function were serum albumin, serum bilirubin, and eGFR. However, on multivariate analysis, only a lower serum bilirubin was significantly associated with the attainment of Child A status after treatment.
The excellent efficacy and safety profile of DAAs has made antiviral therapy possible for patients with advanced liver disease. Treatment in such patients should be individualized, particularly if LT is an available option. In such cases, the challenge is to decide on therapy before or after LT.
In patients with decompensated cirrhosis, who are candidates for LT but access to it is difficult, DAA can be considered as the first line of therapy. Moreover, in patients who are LT eligible and have access to LT, but their MELD score is < 15, treatment with DAA should be considered before LT [21]. Decompensated cirrhotics with a MELD score > 20 and/or eGFR < 30 ml/min/1.73 m2 should undergo liver transplant followed by DAA therapy as they have a lesser degree of improvement in hepatic function after HCV therapy, lower SVR rates (as low as 25%) with limited safety profiles for DAAs [22,23].
Existing data suggest that successful HCV therapy is associated with improvement of hepatic function in 20-60%, which may lead to the delisting of patients (MELD < 16, 16-20, > 20 have a delisting probability of 35%, 12%, 5% respectively) [24,25]. For the past twenty years, HCV has dominated transplant activity in North America and Europe, but with the availability of DAAs the trend is changing. As highlighted in the study by Belli and colleagues from the European Liver Transplant Registry (ELTR), listings for HCV declined from 22% in 2007 to 17% in 2017 [26]. These trends are similar to those of the United Network for Organ Sharing (UNOS), the equivalent registry in the US, where the percentage of patients with a diagnosis of HCV who were on the LT waiting list decreased from 37% in 2012 to 24% in 2016 [27]. So, it can be rightly said that the reign of the HCV as the “king” in liver transplantation is now over, with alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) ready to assume the title! Much of the credit goes to DAA therapy for this achievement.
Dultz et al. in their retrospective analysis of 68 patients of HCV-associated advanced liver cirrhosis on antiviral therapy with pegylated interferon and ribavirin, reported decompensating events during follow-up in 36.8% of cases [28]. On multivariate analysis, MELD score was independently associated with hepatic decompensation (OR = 1.56, 1.18-2.07, p = 0.002). When the patients were grouped according to their baseline MELD scores, hepatic decompensation occurred in 22%, 59%, and 83% of patients with a MELD score of 6-9, 10-13, and ≥ 14, respectively. Baseline factors that were significantly associated with attainment of a compensated state, i.e. Child-Pugh class A, were serum ALT and MELD score before starting DAA therapy (p < 0.05).
In our study, the number of patients with a MELD score > 15 decreased from 21% to 14.5% after treatment with DAA. Two patients who failed to achieve SVR12 after DAA therapy had a MELD score > 15, and they also had decompensating events in the post-treatment follow-up period.
We found that a majority of patients had a BE3A score of 2 (51.6%), followed by 3 (25.8%), 1 (19.4%) and 4 in 3.2%. All patients with a BE3A score of 4 had an improvement in liver function and attained a compensated state after treatment, i.e. CTP class A. However, 15/16 (93.7%) patients with a BE3A score 3, 14/32 (43.7%) with a score of 2 and only 3/12 (25%) with a score of 1 had Child-Pugh class A after treatment with DAA. El-Sherif et al. found that a BE3A score of 4-5 was associated with a 75% chance of achieving CTP A, while those with a score of 0 or 1 were associated with a < 5% and 25% chance of achieving CTP A at 36 weeks, respectively [16]. BE3A scores of 3 and 4 were associated with 87.0% and 98.2% specificity of achieving a sustained CTP A status, whereas BE3A scores of 1 and 0 were associated with 92.3% and 99.6% specificity of not achieving a CTP A status.
A comparison of our composite model with BE3A score using ROC analysis revealed better results with our model, with an AUC value of 0.841 and 0.771 respectively. Our devised model takes into consideration demographic, clinical and biochemical parameters of all the study population, unlike the BE3A score, which used only clinical and biochemical variables. The sensitivity, NPV, and accuracy of our model were better than the BE3A score, whereas the latter had a better specificity and PPV in predicting response to therapy in this population. Moreover, using BMI as a parameter for predicting response, as used in the BE3A score, has serious implications, especially in patients with decompensated cirrhosis with huge ascites with pedal edema. In addition, BMI did not significantly alter the response to DAA in our population.
Dunn et al. in their recent prospective analysis of a similar group of patients found that the BE3A score failed to show significant benefit in predicting response to DAA therapy [29]. The authors found that PNPLA3 CG/GG genotypes could identify a subgroup of patients with decompensated HCV cirrhosis who had suboptimal clinical recovery despite achieving SVR. Genetic polymorphism might be associated with hepatic steatosis (rs738409 of PNPLA3) that may affect biochemical (MELD score) and clinical (CTP score) recovery following DAA treatment for HCV cirrhosis.
There are a few limitations to our study. Being a single-center study, the sample size is small. Moreover, a study with a much longer follow-up period might show a different perspective of the disease. A previous long-term study with sofosbuvir and ribavirin in patients with HCV cirrhosis and portal hypertension showed significant reductions in portal pressure only at 96 weeks after treatment [30]. Lens et al. demonstrated continual improvements in portal hypertension for up to 5 years after therapy, suggesting that a longer time interval is required for hepatic remodeling [31].
Direct or indirect assessment of change in liver fibrosis status in the post-treatment period was not quantified. As all our patients had ascites before starting therapy, baseline transient elastography was not performed. However, other indirect methods of fibrosis determination such as Acoustic Radiation Force Impulse (ARFI) can be considered in future studies for comparison of fibrosis in the pre- and post-treatment period. Lastly, our center lacked linkage to a transplant registry.
Conclusions
The present study shows that in patients with decompensated HCV cirrhosis of CTP class B and C, sofosbuvir-based combination DAA therapy with daclatasvir, ledipasvir or velpatasvir with or without ribavirin, is very effective and often leads to a remarkable clinical improvement with an excellent SVR rate of 98.6%. It also attained compensated status from the baseline decompensated state in more than half of our patients. Our new model performed better than the BE3A score in predicting response to DAA therapy in this population, suggesting that this score can be improved. Moreover, our study showed that patients with a CTP score > 9 and a MELD score > 15 did not respond well to DAA therapy, and we suggest early LT followed by DAA therapy in such patients. However, long-term studies are encouraged before drawing a final conclusion. It should be remembered that such patients are always at risk of developing HCC and screening should be continued as per guidelines. Long-term benefits of DAA therapy need to be established.