Introduction
Acne vulgaris is a very common and devitalising medical condition with complex pathogenesis, and approximately 85% of the population age group (12–24 years of age) experience at least one episode of acne [1–3]. Despite the fact that its predominance diminishes with age, acne can persist throughout adulthood and is known to influence mental and psychosocial wellbeing, particularly when critical scarring is involved [4, 5]. According to the European evidence-based (S3) guideline for the treatment of acne [6], certain topical and systemic treatment options remain a standard. However, novel treatment options are required.
Among systemic treatments, oral antibiotics such as tetracyclines are considered first-line therapies according to the recommendations of the American Academy of Dermatology (AAD) [7, 8]. For numerous decades, isotretinoin, a systemic retinoid, has defended its role as a successful tool in combatting the distortion related to moderate-to-severe acne. The decision to initiate treatment with isotretinoin is often made due to the disappointment with other systemic treatments. Concurrent use of tetracyclines and isotretinoin is known to cause pseudotumor cerebri (PTC) [9]. This disorder of intracranial hypertension is characterized by headaches and visual disturbances, which can lead to visual impairment if the causative agent is continued.
Several studies have analysed the prescription patterns of acne treatments [10–14]. As per the recent analysis, co-prescription of isotretinoin and tetracyclines was rare [1]. But, in one study, isotretinoin in combination with doxycycline on alternate days was found to be efficacious and safe [15]. Although a combination of oral minocycline and isotretinoin is not recommended, topical minocycline gel 4% was found to be 765 times less absorbed systemically; hence, all systemic adverse events associated with oral minocycline were nullified by topical minocycline [16].
Aim
The objective of this study was to investigate the safety and efficacy of the combination of topical minocycline gel 4% and oral isotretinoin and compared against isotretinoin only in the management of acne vulgaris in the Indian population.
Material and methods
Study design
An open-label, prospective, randomised, double-arm clinical study was conducted to assess the efficacy, safety, and tolerability of topical minocycline gel 4% plus isotretinoin (Mino-Iso) against isotretinoin alone (Iso) in Indian patients with moderate-to-severe acne vulgaris. After obtaining written informed consent, patients were screened and eligible patients with acne vulgaris were enrolled in the study. The demographic information and medical history of the patients were recorded. The patients were scheduled to visit every 4 weeks up to week 12. For efficacy evaluations, acne lesion counts (inflammatory, non-inflammatory, and nodulocystic lesions) and investigator’s global assessment (IGA) were performed at baseline and at weeks 4, 8, and 12 with the aim of determining changes in all lesion counts from baseline. The IGA scale, which involves a grading system ranging from 0 (clear), 1 (almost clear), 2 (mild severity), 3 (moderate severity), to 4 (severe), was used for the assessment. Treatment success was defined as an improvement of at least 2 grades in the IGA score from baseline, along with an IGA score of either 0 or 1. Safety was assessed using local skin tolerability on a 4-point Likert scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe) for patient-reported symptoms and those recorded by the treating physician.
Participant’s eligibility criteria
The study included patients aged ≥ 12 years, of either sex, diagnosed with acne vulgaris, who agreed to abstain from using any other acne medication or medicated cleansers, agreed to refrain from excessive sun exposure during the study period, and were willing to participate. Patients with severe systemic diseases, facial sunburn, pregnancy and lactation, allergy to tetracycline-class drugs, clinically significant hepatic impairment, or use of systemic antibiotics within 28 days of screening were excluded from the study. Sixty patients diagnosed with acne vulgaris who met the eligibility criteria were randomised in a 1 : 1 ratio to the treatment groups using block randomisation with a block size of two. All patients were instructed to adhere to a predefined visit schedule as outlined in the study protocol.
Drug administration
In the Mino-Iso group, topical minocycline was administered once a day in the evening. Patients were instructed to use a small amount of topical gel and apply it to the areas of the face affected by acne. This process was repeated until all the acne-affected parts of the face were treated. For acne present in other parts of the body (neck, shoulders, arms, back, or chest), the patients applied an additional amount of topical gel. The patients were asked not to bathe, shower, or swim for at least 1 h after the application of the product. Similarly, all patients were administered isotretinoin capsules (20 mg) once daily, in the evening, after meals. Patient compliance was assessed by collecting and inspecting empty tubes of topical gel and empty strips of capsules at each visit.
Safety and efficacy endpoints
The primary endpoint of the study was to compare the safety and tolerability of a combination of topical minocycline gel 4% and oral isotretinoin with oral isotretinoin in Indian patients with moderate-to-severe acne vulgaris. The safety endpoint comprised treatment-related adverse events leading to treatment discontinuation and local skin tolerability (erythema, dryness, hyperpigmentation, skin peeling, and pruritus). The secondary endpoint of the study was to compare the changes in the number of inflammatory, non-inflammatory, and nodulocystic lesions from baseline to weeks 4, 8, and 12 between the two groups. Another primary endpoint was to compare the IGA score and the proportion of IGA success between the two groups at different visits.
Statistical analysis
Continuous parameters, the number of inflammatory, non-inflammatory, and nodulocystic lesions, and their scores were expressed as mean, median, standard deviation, minimum, and maximum, whereas categorical parameters were expressed as frequencies and percentages. The numbers of inflammatory, non-inflammatory, and nodulocystic lesions were compared between the two groups at different time points using the Mann-Whitney U test. The change in the number of lesions from baseline to different time points was compared between the two groups using the Mann-Whitney U test. Analysis of the IGA score was also performed along similar lines. The investigator’s global treatment success and tolerability assessments were compared using Pearson’s χ2 test. All analyses were performed using the SPSS ver. 26.0 (IBM Corp., Armonk, NY, USA) and statistical significance was tested at the 5% level.
Results
Demographic and disease characteristics at baseline
Sixty patients were enrolled in the study from two centres and were equally randomised into two groups. The baseline characteristics of patients in both arms were compared (Table 1). The mean age of patients in the Mino-Iso group was 20.98 ±5.30 years, while that of the Iso group was 20.97 ±5.61 years. The difference in the means between the two arms was not statistically significant. Similarly, the mean duration of acne, number of inflammatory, non-inflammatory, and nodulocystic lesions, and IGA score did not differ significantly between the two arms, suggesting a homogeneous distribution.
Table 1
Descriptive statistics for demographic and disease characteristics of patients at baseline in the two treatment groups
| Characteristics | Treatment | P-value | ||
|---|---|---|---|---|
| Isotretinoin + topical minocycline gel (N = 30) | Isotretinoin (N = 30) | |||
| Age [years]1 | 20.98 ±5.30; 20 (14, 34) | 20.97 ±5.61; 19 (14, 35) | 0.49† | |
| Gender2 | Male | 9 (30%) | 15 (50%) | |
| Female | 21 (70%) | 15 (50%) | ||
| Grade of acne2 | 3 | 19 (63.3%) | 23 (76.7%) | |
| 4 | 11 (36.7%) | 7 (23.3%) | ||
| Duration of acne [months] | 7.47 ±4.22; 6.5 (3, 18) | 7.63 ±4.71; 7.5 (2, 18) | 0.33† | |
| No. of inflammatory lesions | 32.09 ±7.06; 35 (18, 45) | 30.30 ±8.12; 29 (14, 45) | 0.17‡ | |
| No. of non-inflammatory lesions | 41.20 ±8.96; 42 (26, 58) | 40.67 ±9.28; 38 (12, 55) | 0.37‡ | |
| No. of nodulocystic lesions | 3.63 ±1.00; 4 (1, 5) | 2.97 ±1.03; 3 (1, 5) | 0.24‡ | |
| IGA score | 3.43 ±0.50; 3 (3, 4) | 3.30 ±0.47; 3 (3, 4) | 0.38‡ | |
Efficacy analysis
All 60 patients recruited completed the study. The number of inflammatory, non-inflammatory, and nodulocystic lesions was compared between the two groups at each visit (Table 2). At baseline, the median number of inflammatory lesions in the Mino-Iso group was higher than that in the Iso group; however, the difference was not statistically significant. However, at week 12, the median value was significantly lower in the Mino-Iso group than in the Iso group (p = 0.01). The change in the number of inflammatory lesions from baseline to week 12 was statistically significant in both treatment groups (p < 0.0001). The percentage reduction in the number of inflammatory lesions from the baseline to different visits was obtained, as illustrated in Figure 1. The percentage change was higher in the Mino-Iso group than in the Iso group for all visits. At week 12, the percentage change in the Mino-Iso group (–88.5%) was significantly higher than in the Iso group (–67.42%) (p < 0.05).
Table 2
Comparison of inflammatory, non-inflammatory and nodulocystic lesions between the two groups
| Parameter | Visit | Treatment | P-value2 | |||||
|---|---|---|---|---|---|---|---|---|
| Isotretinoin + topical minocycline gel (n = 30) | Isotretinoin (n = 30) | |||||||
| Mean | SD | Median | Mean | SD | Median | |||
| Number of inflammatory lesions | Baseline | 32.90 | 7.06 | 35 | 30.30 | 8.12 | 29 | 0.17 |
| Week 4 | 23.03 | 8.79 | 20 | 22.33 | 10.55 | 19.5 | 0.52 | |
| Week 8 | 13.27 | 10.80 | 8 | 15.67 | 12.45 | 10 | 0.35 | |
| Week 12 | 6.03 | 8.90 | 0 | 9.87 | 12.20 | 2 | 0.01 | |
| P-value1 | < 0.0001 | < 0.0001 | ||||||
| Number of non-inflammatory lesions | Baseline | 41.20 | 8.96 | 42 | 40.67 | 9.28 | 38 | 0.37 |
| Week 4 | 29.23 | 11.46 | 27 | 30.90 | 12.27 | 25.5 | 0.81 | |
| Week 8 | 18.13 | 13.15 | 12 | 20.87 | 15.56 | 12 | 0.56 | |
| Week 12 | 9.43 | 11.67 | 3 | 14.17 | 16.10 | 4 | 0.06 | |
| P-value1 | < 0.0001 | < 0.0001 | ||||||
| Number of nodulocystic lesions | Baseline | 3.63 | 1 | 4 | 2.97 | 1.03 | 3 | 0.24 |
| Week 4 | 2.03 | 0.76 | 2 | 1.83 | 0.95 | 2 | 0.48 | |
| Week 8 | 0.90 | 0.92 | 1 | 1.13 | 0.97 | 1 | 0.36 | |
| Week 12 | 0.17 | 0.38 | 0 | 0.37 | 0.61 | 0 | 0.34 | |
| P-value1 | < 0.0001 | < 0.0001 | ||||||
Figure 1
A – Percentage reduction in the number of inflammatory lesions, B – percentage reduction in the number of non-inflammatory lesions, C – percentage reduction in the number of nodulocystic lesions
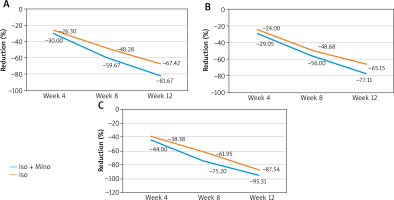
Similarly, the number of non-inflammatory and nodulocystic lesions was compared between the two groups at different visits. From week 4 onwards until the end of the study, the median number of lesions was significantly lower in both groups (p < 0.05). Furthermore, the percentage reduction in the number of non-inflammatory and nodulocystic lesions from baseline to different visits was significantly lower in both groups (Figure 1). However, there was no statistically significant difference between the two groups in terms of the median number of lesions and percentage reduction in lesions.
The IGA scores were compared between the two groups at different visits (Table 3). At the end of the study, there was a statistically significant difference in the median score between the two groups (p = 0.005), whereas for other visits, the difference was not significant. The reduction in the score from baseline to week 12 was statistically significant in both groups (p < 0.0001). Similarly, the investigator’s global assessment of treatment success was significantly higher in the Mino-Iso group than in the Iso group at week 12 (p = 0.03) (Table 3). There were 27 (90%) patients in the Mino-Iso group and 19 (63.3%) in the Iso group who either cleared or almost cleared the acne by week 12.
Table 3
Comparison of investigator’s global assessment parameters between two groups
| Parameter | Visit | Treatment | P-value2 | |||||
|---|---|---|---|---|---|---|---|---|
| Isotretinoin + topical minocycline gel (N = 30) | Isotretinoin (N = 30) | |||||||
| Mean | SD | Median | Mean | SD | Median | |||
| Investigator global assessment score | Baseline | 3.43 | 0.50 | 3 | 3.30 | 0.47 | 3 | 0.38 |
| Week 4 | 2.60 | 0.50 | 3 | 2.63 | 0.49 | 3 | 0.83 | |
| Week 8 | 1.67 | 0.66 | 2 | 1.97 | 0.81 | 2 | 0.18 | |
| Week 12 | 0.47 | 0.68 | 0 | 1.07 | 0.78 | 1 | 0.005 | |
| P-value1 | < 0.0001 | < 0.0001 | ||||||
| n (%) | n (%) | P-value3 | ||||||
| Investigator’s Global Assessment – treatment success at (YES) | Baseline | 0 (0) | 0 (0) | |||||
| Week 4 | 1 (3.3) | 0 (0) | 1 | |||||
| Week 8 | 19 (63.3) | 18 (60.0) | 1 | |||||
| Week 12 | 27 (90) | 19 (63.3) | 0.03 | |||||
Safety and tolerability assessment
Eleven adverse events were reported in this study. In the Mino-Iso group, 6 patients reported 7 AEs, whereas 4 patients reported 4 adverse events (AEs) in the Iso group (Table 4). This difference was not statistically significant (p = 0.73). One patient in the Mino-Iso group had a headache, for which fundoscopy was performed, which revealed no abnormalities. Moreover, all AEs were mild and resolved over the course of the study. None of the patients discontinued the study owing to AE.
Discussion
Acne significantly impacts physical appearance and thus, the individual’s quality of life [12]. As acne severity increases, the quality of life is expected to deteriorate [17–19]. Nodulocystic acne, a severe form of acne vulgaris, has psychological and social implications [15, 20, 21]. Timely and effective treatment of nodulocystic acne can prevent physical scarring and emotional distress. However treating severe nodulocystic acne is becoming increasingly challenging and may necessitate a combination of medications [21]. Despite the availability of multiple acne treatment methods, there is no consensus on the best approach [22].
Systemic treatment of severe acne typically involves isotretinoin and oral antibiotics such as minocycline, which is considered the standard approach. However, combining these two treatments is not recommended due to the potential for increased adverse effects [23]. As a safer alternative, topical formulations of minocycline show promise in treating acne while minimizing the serious side effects associated with oral minocycline owing to lower systemic absorption [24]. Hence, this study was designed to evaluate the safety and efficacy of a combination of topical minocycline gel 4% and oral isotretinoin and compare it against isotretinoin only in the management of acne vulgaris in Indian population.
Starting right from week 4 onwards, the combination of topical minocycline and oral isotretinoin resulted in a better reduction in inflammatory lesions than oral isotretinoin, which was statistically significant at the end of the therapy. However, in terms of other lesions, such as non-inflammatory and nodulocystic lesions, there was no statistically significant difference between the two groups. This demonstrated the anti-inflammatory properties of minocycline. Minocycline reduces the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. These anti-inflammatory and antioxidant properties, along with the inhibition of collagenase, are crucial for managing acne [25, 26]. Furthermore, minocycline has greater efficacy against C. acnes, possibly because of its high lipophilicity, enabling it to attain higher concentrations in sebaceous follicles than other tetracyclines [27]. These advantages of minocycline led to significantly better results than isotretinoin in terms of IGA success. By the end of treatment, 90% of the patients in the minocycline and isotretinoin group achieved IGA treatment success compared to only 63% in the isotretinoin group.
The simultaneous use of minocycline and isotretinoin is known to cause PTC [9]. Research indicates that minocycline itself may have the potential to induce PTC, and there have been reports of individuals developing PTC symptoms when using isotretinoin and tetracycline [28, 29]. Studies conducted by Moskowitz et al. and Tintle et al. recommended regular ophthalmic examinations and careful monitoring for PTC in patients taking oral minocycline in combination with vitamin A derivatives [30, 31]. According to data from the National Ambulatory Medical Care Survey (NAMCS), co-prescription of isotretinoin and tetracycline was uncommon and observed in only a small fraction of all acne-related visits [32].
In our study, this combination was safe and tolerable, with no statistically significant differences between the two groups. Only 1 patient in the topical minocycline + isotretinoin group complained of headache, but fundoscopy revealed no abnormalities. This suggests that topical minocycline may be safe in combination with isotretinoin, owing to its lower systemic exposure. However, monitoring is recommended to rule out early adverse events, as recommended by Kohli et al. in their review [33].
Conclusions
To the best of our knowledge, this is the first comparative clinical study to examine the efficacy, safety, and tolerability of a combination of topical minocycline gel 4% and oral isotretinoin compared with oral isotretinoin alone in the management of moderate-to-severe acne. The results of this study indicated a statistically significant improvement in the IGA score and success rate in the topical minocycline with isotretinoin and isotretinoin groups over 12 weeks. Moreover, inflammatory lesion counts were significantly reduced in the topical minocycline with isotretinoin group compared to the isotretinoin group. In terms of safety, the combination of topical minocycline and oral isotretinoin was well tolerated and there was no significant difference between the two groups. Hence, a combination of topical minocycline 4% and oral isotretinoin is preferable for the treatment of individuals with moderate-to-severe acne.








