Introduction
Atopic dermatitis is a commonly chronic and relapsing inflammatory skin condition with immune dysfunction affecting lesional and non-lesional skin resulting in intense pruritus [1–3]. It has the features of pruritus, skin pain, eczematous lesions, and dry skin [4–6]. Many mechanisms participate in this pathophysiology, and they include impaired skin barrier function, immune dysregulation, genetic susceptibility, and environmental factors [7–9]. This disease results in considerable impairment in quality of life, sleep, depression, anxiety, and work absenteeism [10–12].
Current treatments are still ineffective for some patients with atopic dermatitis [13–15]. As an oral Janus kinase (JAK) 1 selective inhibitor, abrocitinib has potential in treating atopic dermatitis. For instance, abrocitinib was effective and well tolerated in adults with moderate to severe atopic dermatitis, as shown by the improvement in Investigator Global Assessment (IGA) response and Eczema Area and Severity Index (EASI) score [16]. Another phase 3 trial of abrocitinib (200 mg or 100 mg) also demonstrated benefit for the treatment of moderate to severe atopic dermatitis [17].
Aim
However, the efficacy of abrocitinib 100 mg versus 200 mg for atopic dermatitis has not been well established, and conflicting results are seen [17–19]. This meta-analysis of RCTs is intended to explore the efficacy of abrocitinib 100 mg versus 200 mg for patients with atopic dermatitis.
Material and methods
This meta-analysis was conducted in adherence to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). Ethical approval and patient consent were not required because this was a meta-analysis of previously published studies [20].
Search strategy and study selection
We searched the following databases from inception to July 2021: PubMed, EMbase, Web of science, EBSCO, and the Cochrane Library. The keywords for electronic search strategy were “abrocitinib” AND “atopic dermatitis”. We also checked the reference lists of the screened full-text studies to identify other potentially eligible trials.
The inclusive selection criteria were as follows: (i) study design was RCT; (ii) patients were diagnosed with atopic dermatitis; and (iii) abrocitinib was administered at the dose of 200 mg versus 100 mg once daily.
Data extraction and outcome measures
The following information was extracted: author, number of patients, age, sex, duration of atopic dermatitis, Eczema Area and Severity Index (EASI) score, and detail methods in each group, etc. Data were extracted independently by 2 investigators, and discrepancies were resolved by consensus. The primary outcomes included IGA response and EASI-75. Secondary outcomes included NRS response, adverse events, and serious adverse events.
Quality assessment in individual studies
We independently assessed the methodological quality of the included studies by the modified Jadad scale [21]. There were three items for the Jadad scale: randomisation (0–2 points), blinding (0–2 points), and dropouts and withdrawals (0–1 points). The score of the Jadad Scale varied from 0 to 5 points. Jadad score ≤ 2 suggested low quality, while Jadad score ≥ 3 suggested high quality [22].
Statistical analysis
The odds ratio (OR) with 95% CI was measured for all dichotomous outcomes. The random-effects model was used regardless of heterogeneity. Heterogeneity was reported using the I 2 statistic, and I 2 > 50% indicated significant heterogeneity [23]. Whenever significant heterogeneity was present, we searched for potential sources of heterogeneity by omitting one study in turn for the meta-analysis or performing subgroup analysis. All statistical analyses were performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Literature search, study characteristics, and quality assessment
Figure 1 shows a detailed flowchart of the search and selection results. Initially, 164 potentially relevant articles were identified, and 4 RCTs were finally included in the meta-analysis [16–19]. The baseline characteristics of the 4 eligible RCTs are summarized in Table 1. The 6 studies were published between 2019 and 2021, and the sample size ranged from 111 to 464 with a total of 1198. The intervention treatments were 200 mg versus 100 mg of abrocitinib once daily for 12 weeks.
Table 1
Characteristics of included studies
Among the 4 studies included herein, 4 reported Investigator’s Global Assessment (IGA) response and EASI-75 [16–19], 3 reported Numerical Rating Scale (NRS) response [16, 17, 19], and 3 reported adverse events and serious adverse events [17–19]. Jadad scores of the included studies varied from 4 to 5, and thus they were considered to have high quality according to quality assessment.
Primary outcomes: IGA response and EASI-75
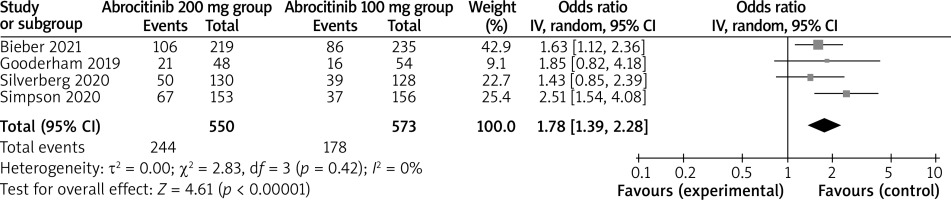
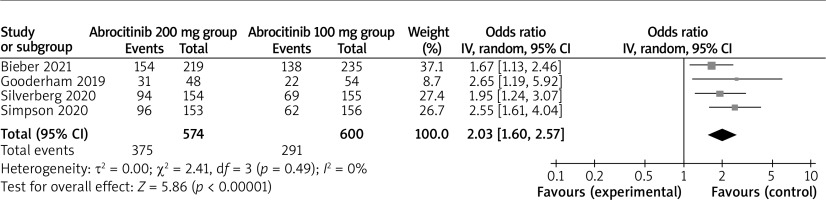
Compared to the abrocitinib 100 mg group for atopic dermatitis, the abrocitinib 200 mg group had substantially higher IGA response (OR = 1.78; 95% CI: 1.39–2.28; p < 0.00001) with no heterogeneity among the studies (I2 = 0%, heterogeneity p = 0.42) (Figure 2) and EASI-75 (OR = 2.03; 95% CI: 1.60–2.57; p < 0.00001) with no heterogeneity among the studies (I2 = 0%, heterogeneity p = 0.49) (Figure 3).
Sensitivity analysis
No heterogeneity was seen among the included studies, and thus we did not perform sensitivity analysis by omitting one study in turn.
Secondary outcomes
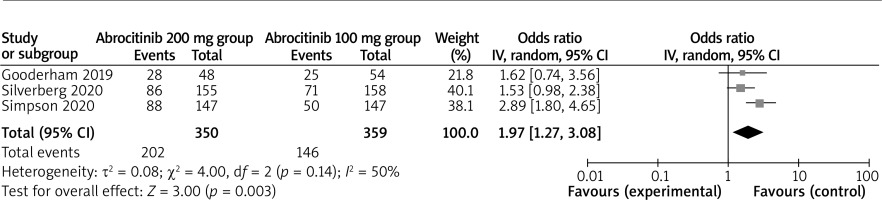
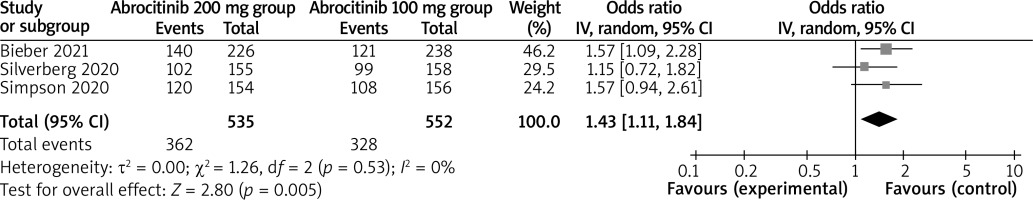
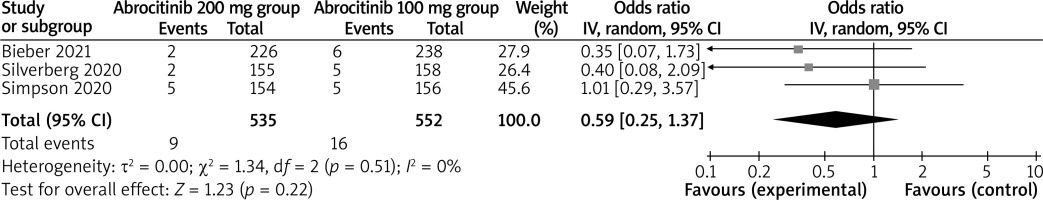
In comparison with the abrocitinib 100 mg group for atopic dermatitis, the abrocitinib 200 mg group was associated with improved NRS response (OR = 1.97; 95% CI: 1.27–3.08; p = 0.003; Figure 4) and adverse events (OR = 1.43; 95% CI: 1.11–1.84; p = 0.005; Figure 5), but revealed no significant impact on serious adverse events (OR = 0.59; 95% CI: 0.25–1.37; p = 0.22; Figure 6).
Discussion
In patients with moderate to severe atopic dermatitis, short-term systemic corticosteroids is widely accepted because it has greater efficacy than topical treatments. However, systemic corticosteroids resulted in short-term and long-term side effects [24]. Immunosuppressive drugs such as cyclosporin, methotrexate, and azathioprine have revealed some promise in atopic dermatitis, but they are not approved because of adverse events and poor tolerability [24]. Abrocitinib is known as an oral Janus kinase (JAK) 1 selective inhibitor, and its monotherapy is associated with improved outcomes for atopic dermatitis [16, 17, 19].
In particular, the 2 doses of abrocitinib (200 mg and 100 mg once daily) are commonly used for atopic dermatitis, but their efficacy and safety are not well established [17–19]. Our meta-analysis included 4 RCTs and 1198 patients with atopic dermatitis. The results revealed that 200 mg abrocitinib was associated with better IGA response, EASI-75, and NRS response than 100 mg abrocitinib for these patients. This suggests that the efficacy of abrocitinib improved in a dose-increasing manner. Its benefits act mainly via inhibiting signalling of interleukin-4, interleukin-13, and other cytokines involved in the pathogenesis of atopic dermatitis [25].
In terms of adverse events, our meta-analysis demonstrated similar incidence of serious adverse events between 200 mg abrocitinib and 100 mg abrocitinib, but 200 mg abrocitinib resulted in the increased incidence of total adverse events compared to 100 mg abrocitinib. The increased adverse events mainly include nausea, headache, and vomiting, which are all generally mild and acceptable [17, 19]. JAK inhibition may increase the risk of infections due to the involvement of JAK signalling pathways that regulate the host defence and immune response [26]. However, abrocitinib revealed a less immunogenic response than biologic treatment [27]. The incidence of serious infections and herpes virus infections was low, and no malignancy was seen [19].
Several limitations exist in this meta-analysis. Firstly, only 4 RCTs were included, and more RCTs are needed to compare the efficacy and safety of 200 mg abrocitinib and 100 mg abrocitinib for atopic dermatitis. Secondly, there was short duration of treatment and follow-up, which does not address the long-term efficacy and safety of 200 mg abrocitinib versus 100 mg abrocitinib. Thirdly, no significant heterogeneity remains during the sensitivity analysis, but different severity levels of atopic dermatitis and age ranges may produce some bias.