Direct immunofluorescence: past
RH Cormane was the first to report, in an article based on a work presented at the First Congress of the European Society of Pathology, Warsaw, Poland, 1–3 June, 1966, detecting granular immunoglobulin deposits at the dermal-epidermal junction (DEJ) using direct immunofluorescence (DIF) of uninvolved dermatitis herpetiformis (DH) skin [1]. Later, JB van der Meer, a mentee of Cormane, reported that he was able to find granular IgA deposits in uninvolved DH skin with DIF [2]. It was even reported that IgA deposits with DIF can be found in oral mucosa in a fraction of DH patients showing cutaneous involvement with no gingival lesions [3] hinting that IgA deposition at uninvolved sites distant from lesions may be an epiphenomenon.
Direct immunofluorescence: present and future
The cost-effective DIF should still be regarded as a crucial procedure in diagnosing DH at the laboratory level, i.e. it is necessary to perform it in any individual suspected to have DH. Perilesional, uninvolved skin, similarly to other autoimmune blistering dermatoses showing cutaneous lesions, is the optimal biopsy site for DIF [4] as this approach minimizes the risk of obtaining a false negative result reducing the need for the biopsy repetition. The tissue for DIF should be processed according to the established methodology; it is vital not to use formalin fixation and paraffin embedding.
DIF can be visualized with short arc mercury lamp-operated microscopy, blue light-emitting diode technology-operated microscopy and laser scanning confocal microscopy [5] (Figure 1). Conceptually, a super/high resolution microscopy technique known under the acronym STED (stimulated emission depletion) [6], if adapted for the routine laboratory use, should also be useful for evaluation DIF images, especially as nowadays it can be fixed to the microscope of any type utilizing just the shoebox size equipment.
Figure 1
Microgranular IgA deposits at the tips of the dermal papillae in a young female with DH in DIF of perilesional skin visualized with blue light-emitting diode technology-operated microscopy (A). Microgranular IgA deposits at the tips of the dermal papillae in a young female with DH in DIF of perilesional skin visualized with laser scanning confocal microscopy (B). Simultaneous, in a single section, microgranular IgA deposits at the tips of the dermal papillae, microgranular-fibrillar deposits at the tips of the dermal papillae and microgranular deposits along the DEJ in a young male with DH in DIF of perilesional skin visualized with short arc mercury lamp-operated microscopy (C). Microgranular IgA deposits along the basement membrane of a hair follicle in a young male with DH in DIF of perilesional skin visualized with short arc mercury lamp-operated microscopy (D) (original objective magnifications 40×)
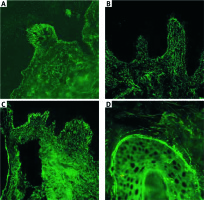
With DIF the following three main patterns of IgA deposition can be seen, namely microgranular deposits at the tips of the dermal papillae, microgranular-fibrillar or just fibrillar deposits at the tips of the dermal papillae and microgranular deposits along the DEJ [7–9]. However, with serial sectioning of the tissue as many as seven patterns can be seen since grouping (Figure 1) of three main patterns is possible (in mathematics this is the concept of the power set but excluding the empty set) [7]. Japanese authors have recently dubbed the variety of DH with a fibrillar pattern in an apt way as fibrillar-type DH showing that those deposits are co-localizing with fibrinogen which is in agreement with non-fibrillar types of DH and can have pathological importance as epidermal transglutaminase tends to be translocated from its physiological expression sites and also deposited at sites of IgA deposition [10, 11]. In an earlier study, one-third of Japanese DH patients showed fibrillar IgA deposition [12]. This should be regarded as an unusually high frequency since that pattern was detected in merely 9% (5 of 54) of Polish DH patients [13]. Initially, there were data that in all 8 DH cases studied both IgA1 and IgA2 were forming IgA cutaneous deposits seen with DIF, although IgA1 predominated in them [14]. Later, we detected IgA1 (found in all 24 DH cases) and IgA2 (found in 4 of 13 DH cases) cutaneous deposits with DIF concluding that IgA1 deposition was far more frequent and intense compared to that of IgA2 [13]. What may be important from the diagnostic point of view, in certain of our DH cases testing for IgA1 gave less background staining compared to IgA making the interpretation of imaging results easier. It was reported that IgA deposits can disappear from the papillary dermis of patients with DH after a long-term gluten-free diet [15].
In addition to IgA deposition in the close vicinity of the DEJ, IgA was reported in certain patients with DH to be present in the vessels of the papillary dermis and in the subpapillary vasculature [16] of the reticular dermis. Even less frequently, IgA is detectable in the elastic fibers, in the arrector pili muscles, in the fibers around hair follicles and in the basement membrane of sweat glands and ducts [17]. Microgranular IgA deposits can occasionally be detected along the basement membrane of the hair follicles (Figure 1). The value of such findings for diagnosing DH is uncertain; they may simply reflect either the physiologically dynamic expression of DH antigens (epidermal and tissue transglutaminases) in the tissue or the traffic of those antigens through various structures of the skin affected by DH pathology or both. Interestingly in this respect, codistribution of tissue transglutaminase and tissue-bound IgA in jejunum of patients with DH was reported in the meeting abstract [18].
IgA deposits at the DEJ of perilesional skin with DIF can also be found in coeliac patients with inflammatory skin diseases different from DH and, therefore, could even be considered as a marker of coeliac disease [19]. Interestingly, analysing 16 DH patients with both ELISA and immunoblot, we noticed certain discrepancies between the results of DIF and testing for circulating autoantibodies incriminated to be DH serum biomarkers. Specifically, according to the interpretation of Cohen’s κ, the inter-rater agreement/reliability between DIF and ELISA as well as immunoblot detecting antibodies to tissue transglutaminase and nonapeptides of gliadin was weak [20]. There are some DH patients described in the literature with negative DIF [21]. Probably, as suggested by Sousa et al., technical errors, failure of current laboratory methods in detecting cutaneous IgA deposits in some patients, and focal deposition of IgA in the skin may explain the negative DIF in DH. Furthermore, it was reported that there are female DH sufferers having the positive DIF in whom hypersensitivity to nickel detected with epicutaneous patch tests with 5% nickel sulfate hexahydrate in Vaseline can be found [22]. Putting aside the issue whether in certain females DH is pathologically related to nickel hypersensitivity, this coincidence definitely widens the burden of skin problems in such individuals [23]. Thus, expanding on initial ideas of Beutner et al. [24], thoughtful consideration of all clinical as well as imaging and molecular-biochemical laboratory data available is mandatory to establish the diagnosis of DH.
With DIF techniques, DH bodies detectable in the papillary dermis of affected DH skin were found to be comprised of an amalgamation of IgA, IgM as well as armadillo repeat gene deleted in velo-cardio-facial syndrome (ARVCF), desmoplakins 1 and 2, and plakophilin 4, but not tissue/epidermal transglutaminase [24]. Pathophysiologic significance of DH bodies is at present virtually unknown, but they may be a sign of an ongoing tissue repair processes. The diagnostic potential of DH bodies remains to be elucidated.
It was reported that in some patients with the rash resembling DH the deposition of exclusively C3 at DEJ was found. These patients may represent a new disease entity, different from DH and plausibly related to the non-coeliac gluten sensitivity [25], but important as far as the laboratory differentiation of DH with imaging DIF is concerned, for which the term “granular C3 dermatosis” was proposed [26]. It should be stressed here that coining catchy terms for apparently newly described dermatoses should be done extremely cautiously as the term “psoriasis bullosa acquisita” [27] was discarded quickly after an initial enthusiasm.








