Introduction
The Anthemisgenus, belonging to the Asteraceae family, is composed of about 210 species [1]. In Cyprus there are 10 species belonging to the Anthemis genus and one of them is endemic Anthemis tricolorBoiss [2, 3]. Traditionally, the Anthemis genus has been used for the treatment of gastrointestinal disorders, haemorrhoid and stomach ache in Europe [4–7]. There are several in-vitro and in-vivo studies that showed the pharmacological properties of Anthemisspecies such as antibacterial [8, 9], anti-inflammatory [10, 11], antiproliferative [12] and antiprotozoal [13] properties. Our previous studies indicated the possible anti-inflammatory effect of A. tricolor [10, 11].
The most commonly used method to investigate the anti-inflammatory potential of topical dermatological preparations is the UV erythema test [14–17]. The preparations are applied to the skin in the form of occlusion with positive and negative controls. Regression power of erythema of the preparation is measured 24 and/or48 h after UVB application [14, 15].
Aim
Our study used the model of inflammation formed in rats; we aimed to evaluate anti-inflammatory properties of A. tricolor extracts. The bioactivity and chemical composition studies of A. tricolor have so far been limited and there has been no report on anti-inflammatory activity.
Material and methods
In our study, 5 different extracts of A. tricolor were tested against the negative control and 2 different topical corticosteroids (betamethasone and hydrocortisone).
Plant material
The aerial part of A. tricolorBoiss was collected in March 2013 from the Kalkanlı region of North Cyprus. The material was identified by ÖzgeÖzçınar. A voucher specimen was deposited in the Herbarium of the Faculty of Pharmacy at Ege University, İzmir.
Extraction of plant material
n-Hexane, chloroform and methanol extracts were separately prepared from 150 g of the aerial parts of air-dried and powdered plant material by extracting on a shaker (room temperature/200 rpm) for 48 h (150 g of the plant material/1500 ml of the solvent). The solvents were then removed to dryness under reduced pressure. The yields of n-hexane, chloroform and methanol extracts were 0.38%, 1.42% and 6.4%, respectively.
Preparation of fatty acid extract
The oil extraction of the air dried and powdered aerial parts (10 g) of A. tricolor was carried out at 60°C for 6 h by Soxhlet extractor, using petroleum ether as a solvent. The solvent was evaporated by a rotary evaporator. The fatty acids were esterified into methyl esters by saponification with 0.5 N methanolic NaOH and transesterified with 14% BF3 (v/v) in methanol [10].
Total sesquiterpene lactone extract
Air-dried plant material (40 g) was extracted at room temperature with petroleum ether-Et2O (peroxide-free)-MeOH (1 : 1 : 1 v/v) and evaporated under vacuum to yield a crude extract. This extract was dissolved in a mixture of cyclohexane-Et2O (peroxide-free)-MeOH (1 : 1 : 1 v/v), then brine was added and it was extracted with EtOAc. The organic phase was concentrated under vacuum to dryness and yielded a lipophilic residue (1.2%).
Preparation of test specimens for bioassay
All extracts were suspended in a suspension of 0.5% sodium carboxymethyl cellulose (CMC) in distilled water.
Animals
Forty male Albino Wistar rats weighing 450–500 mg were included in the prospective, randomized, double blind study. The rats were kept under appropriate conditions for moisture and temperature (24 ±2°C). The rats had unlimited access to water and food. The rats were shaved with a razor blade and foam. All study designs and methods were approved by the Kırıkkale University Animal Ethics Committee.
Anti-inflammatory activity model
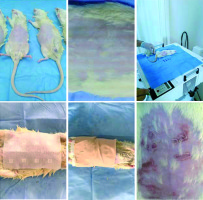
Five fluorescent Philips PLL33W (Philips GmbH, Hamburg, Germany) lamps with the highest wavelength of 310 nm placed on a UV 181 BL (Waldmann GmbH,VS-Schwenningen, Germany) phototherapy device were used for irradiation. UV-B was applied at a gradual incremental dose (0.2 mJ/cm2) to detect a minimal erythema dose (MED) to the shaved abdominal area of the rat. For the Wistar albino rat, MED was found to be 1 mJ/cm2, 1.5-fold MED; each 1 cm2 area was treated with 1.5 mJ/cm2 UVB (Figure 1). The erythema in these areas was measured photometrically using Mexameter (t1) [14]. Material to be tested was administered by a blind applicator in the form of occlusion in a volume of 50 µl to each test site in accordance with the randomization schedule. Patches were removed 47 h after applying the material and an 1 h before evaluating the test areas to prevent false positivity against the patches. Afterwards, the erythemas of all test areas were measured (t2) and photometric documentation was done.
Results
n-Hexane and the total sesquiterpene lactone extract of A. tricolorBoiss significantly reduced erythema compared to the negative control (p< 0.001). The erythema in the area where the n-hexane extract was used was significantly lower than that of the other extracts of A. tricolorBoiss (p< 0.001). The erythema in the area where the n-hexane extract was used was significantly higher than in the areas using betamethasone 17-valerate and hydrocortisone acetate (p< 0.001). Significantly more erythema was found in the areas where the sesquiterpene extract was used than in the area where betamethasone 17-valerate and hydrocortisone acetate were used (p< 0.001), but there was significantly less erythema than methanol and fatty acid extracts (p< 0.001). There was no significant difference between the area where the sesquiterpene extract was used and the area where the chloroform extract was used (p = 0.267). There was no difference in the area between the negative control used area and the areas using methanol, chloroform, fatty acid extract in terms of erythema (p> 0.05). There was no significant difference between betamethasone 17-valerate and hydrocortisone acetate in terms of UVB-induced erythema (p = 0.223). Erythema in areas using betamethasone 17-valerate and hydrocortisone acetate was significantly lower than that in areas using the negative control, chloroform, n-hexane, ethanol, fatty acid extracts (p< 0.001). There was no irritation (erythema) due to patches in the non-irradiated test areas (Table 1).
Table 1
Effects of the preparations on the erythema occurring in 1.5-fold MED UVB-applied areas
Discussion
The UV erythema test is used to measure the anti-inflammatory effect of topically applied substances [14, 15, 18]. It had been first applied to the rats for comparison with the foot oedema test [19]. The UV erythema test should be done with at least 40 samples and the applied UVB should be 1.5-fold MED [14]. It is useful to add corticosteroids such as betamethasone and hydrocortisone as a control substance to evaluate anti-inflammatory activity [14]. In a study of acute and chronic inflammatory models investigating the anti-inflammatory effect of extracts in rats, the extract of A. aciphylla var. aciphylla Boiss was systemically administered; it is reported that this plant is effective in both acute and chronic inflammatory processes [11]. The results of our study showed that only n-hexane and sesquiterpene extracts of A. tricolor have the anti-inflammatory effect. The effects of topically applied herbal extracts differ between themselves due to the differences in the constituents of extracts, tissue penetration and different properties in the physicochemical reactions.
The anti-inflammatory effect of n-hexane and sesquiterpene extract of A. tricolorBoiss is lower than that of betamethasone 17-valerate and hydrocortisone acetate. Corticosteroids are one of the highest anti-inflammatory agents.However, when considering the side effect profile of topical corticosteroids; Boiss’ hexane and sesquiterpene extract of A. tricolor may be a good alternative. Although the UV erythema test is known as the optimal anti-inflammatory model, the anti-inflammatory properties of A. tricolor can be investigated by creating other inflammatory models.
A. tricolor can be investigated by in-vivo tests by establishing a similar model of anti-inflammatory activity in humans to assess anti-inflammatory activity.
Conclusions
This is the first report on anti-inflammatory activity of A. tricolor and we showed the anti-inflammatory effect of n-hexane and sesquiterpene extract of A. tricolor with UV erythema test as a result, A. tricolorBoiss extracts can be used for the treatment of inflammatory skin diseases due to anti-inflammatory activity. Further studies are warranted to investigate the phytochemical composition of these extracts.