The number of patients with severe forms of psoriasis in whom all treatment modalities have failed, including biological therapy, is increasing. The condition of these patients is not satisfactory and physicians in specialized centres are looking with hope at results of clinical studies and the launch of new molecules into routine practice. Secukinumab, an IL-17R inhibitor, is the newest biological agent for routine clinical practice and was approved by the European Medicines Agency for the treatment of psoriasis on 15 January 2015 [1–3].
I describe the case of a young patient with a severe course of arthropathic psoriasis, in whom therapy was associated with a number of complications and whose condition was successfully managed only several years after secukinumab had been started.
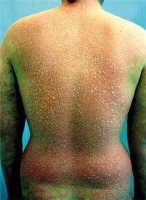
The patient is a 36-year-old man with a history of psoriasis since the age of 28. At the beginning, the disease was manifested by predominant involvement of the face and scalp, extremely resistant to external therapy (Figure 1). Gradually, the lesions progressed and became scattered all over the body and a moderate polyarticular form of psoriatic arthritis with high clinical activity developed 1 year after disease onset. The patient is considerably overweight (116 kg) and he developed diabetes mellitus requiring insulin therapy, hepatic steatosis and resting tremor in the upper limbs during the course of psoriasis treatment. He had a negative family history of psoriasis; the patient’s brother was treated for atopic dermatitis. He had a negative history of drug allergies.
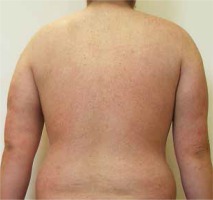
Because the joint manifestations were resistant to external therapy and phototherapy, and there was a high activity of joint inflammation, methotrexate therapy was initiated in cooperation with a rheumatologist, however it had to be discontinued due to marked hepatotoxicity. A diagnosis of severe steatosis was made based on a detailed liver examination and continuous therapy with hepatoprotective agents was initiated. Cyclosporine therapy was insufficiently effective and poorly tolerated, with development of a resting tremor in the upper limbs and to a lesser extent in the lower limbs which partially resolved following drug discontinuation. Etanercept therapy was initiated due to persisting high activity of the skin and joint problems with no response. Hence, the patient switched to adalimumab after 6 months. After 4 weeks of therapy, no improvement of the patient’s condition was observed. On the contrary, a psoriasiform exanthema developed gradually and subsequently turned into erythroderma (Figure 2). A suspected paradoxical drug reaction following adalimumab leading to the induction of erythrodermic psoriasis was confirmed by histological examination. Erythroderma resolved after discontinuation of adalimumab and after several weeks of therapy with methylprednisolone 16 mg/day, cyclosporine 50 mg/day and the application of potent topical corticosteroids (Figure 3). However, the original torpid chronic plaques remained with the same extent and intensity (BSA 14%, PASI 18.2), in addition to joint swelling and stiffness. Hence, therapy with ustekinumab 90 mg s.c. was initiated but had to be discontinued due to inefficacy. The last available treatment alternative was infliximab. Infliximab treatment was initiated in a standard regimen with a dose of 5 mg/kg and remission of skin and joint manifestations was induced following the third infusion. Unfortunately, an allergic reaction with dyspnoea, facial skin and conjunctival hyperaemia and generalized pruritus developed during the 4th infusion. The infusion was immediately stopped and intravenous hydrocortisone and antihistamine agents were administered; the patient’s condition normalized within several minutes. After approximately 4 weeks, there was a relapse, particularly the skin and, to a lesser extent, joint problems occurred and disease activity continued to increase in spite of ongoing therapy with methylprednisolone 8 mg daily and cyclosporine 50 mg daily. Shortly after the marketing authorization of secukinumab, we initiated therapy with this agent with a dose of 300 mg s.c. (at week 0, 1, 2, 3, 4 followed by every 4th week). The induction of disease remission was successful and is maintained at week 24 (PASI score at week 0, 4, 8, 16, 24: 34.4, 33.6, 8.4, 3.6, 3.2, respectively) (Figure 4).
Figure 3
Successful treatment of paradoxical psoriasiform reaction with methylprednisolone, low-dose cyclosporine and corticosteroid ointments
The results of clinical studies evaluating the efficacy and safety of secukinumab are very promising. Efficacy seems to be the highest among all available biologic drugs, however some questions remain unresolved, such as the long-term data as well as secukinumab efficacy following failure of previous biologic drugs, because clinical studies to date have mainly focused on biologic treatment-naive patients [3–6]. Potential for remission induction with secukinumab in subsequent lines of therapy following failure of previous treatments will have the same importance as secukinumab efficacy in treatment-naive patients in routine practice. For a certain group of patients, secukinumab will be the only option after failure of all other alternatives. Several years following the implementation of biologic therapy for treatment of psoriasis, the number of patients who are primary non-responders or secondary-resistant to the given biologic agent is growing. Whereas primary response to treatment is sometimes unsatisfactory due to certain comorbidities or lifestyle (obesity, diabetes or excessive alcohol consumption), secondary treatment failure is most commonly caused by the development of neutralizing antibodies (anti-drug antibodies) and occurs most commonly with infliximab and adalimumab [7–9].
The presented case is a typical example of a complicated patient with many comorbidities, who developed complications during systemic therapy and had a poor therapeutic response to most available agents. A short-term effect was observed only with infliximab, however an allergic reaction resulted in treatment discontinuation. A certain scepticism was also associated with secukinumab therapy initiation and the first 5 weeks of therapy confirmed our concerns. However, a turn in the treatment course occurred in the 8th week and the induced remission is subsequently maintained and well tolerated by the patient. A higher number of treated patients is necessary to conclude whether secukinumab will be a solution for similarly complicated cases. Our positive experience, despite the initial low expectations, is a certain signal that secukinumab could provide a significant benefit at least for a proportion of resistant and complicated cases, due to its slightly different action in the pathogenetic processes of psoriasis.