Introduction
Isotretinoin, the most effective medication in acne treatment these days, exerts its effects through four pathogenic factors. These factors can be summarized as that the medication affects the progression of the cell cycle, cellular differentiation, cell recovery and apoptosis. In this way it causes a significant decrease in sebum production, affects comedogenesis, decreases surface and ductal Propionibacterium acnes, and demonstrates anti-inflammatory effects. Isotretinoin is recommended in treatment of serious forms such as nodules or conglobate acne, when there is a risk of permanent scar formation and when standard treatment with systemic antibiotics and topical treatment are not satisfactory [1].
Isotretinoin is significantly effective; however, it is associated with many side effects which can restrict its usage. The main adverse effect of isotretinoin is known as teratogenicity. Despite the fact that all of them are very rare, the other side effects can be listed as cheilitis, dryness of eye and mouth, photosensitivity, conjunctivitis, hypertriglyceridemia, pancreatitis, increase of serum cholesterol levels and liver enzymes, blood dyscrasia, hyperostosis, early epiphysis closure and nyctalopia [2]. In many recent studies there were no determined negative effects of isotretinoin on cardiac functions [3, 4].
However, there has been no recent study researching the potential effects of isotretinoin on the autonomic nervous system by carrying out SSR analysis.
The sympathetic skin response (SSR) is defined as the electrical potential change occurring on skin after stimulation with any internal or external stimulus. This reflex, which can be stimulated in several ways, uses preganglionic and postganglionic sympathetic sudomotor fibers as an efferent pathway. It reaches the last organ, the sweat glands [5]. It was asserted that the SSR represents sympathetic sudomotor flow in the central and peripheral nervous system. Recently many studies have indicated a potential relation between SSR and autonomic dysfunction, and there are even significant changes in response amplitude and latencies [6–8].
Aim
In this study the effects of the medication on the autonomic nervous system, heart rate and cardiac rhythm were investigated by carrying out SSR analysis and ECG scanning in patients admitted to the dermatology clinic due to acne vulgaris.
Material and Methods
Materials
Thirty patients diagnosed with acne vulgaris were included in the study. The average age of the patients was 20.93 ±5.03 (21.04 ±4.93 for women; 20.62 ±5.62 for men). Twenty-two (77.3%) of the patients were women and 8 (26.7%) were men. In all patients ECG scanning and SSR analysis were performed both before treatment and 1 month after the start of treatment.
None of the patients had any other diseases apart from acne vulgaris. They had received treatment with oral isotretinoin at a dose of 0.5 mg/kg per day for at least 1 month. Patients with diseases such as cardiac disease, diabetes mellitus, hypo/hyperthyroidism, chronic obstructive respiratory disease, congestive cardiac failure, coronary artery disease, alcoholism, collagen tissue disease, atrioventricular transmission abnormalities and people on medications known to change cardiac transmission were excluded from the study.
From all patients informed consent forms were obtained, and before the beginning of the study ethics committee approval was obtained from Harran University.
Electrophysiological study
In all patients SSR analysis was performed before and 1 month after treatment. In our electromyography (EMG) unit SSR analysis was carried out before and 1 month after treatment using Dantec Keypoint EMG Device V2.32. SSR analysis was recorded by placing active electrodes into the palm and surface electrodes on the dorsum of the hand after cleaning the skin of hand and wrist while the patient was lying down. For recording, normal ambient temperature (24–26ºC) was used and the temperature of the hand skin was 30ºC, and when necessary the patient was warmed up. Silver cup electrodes were used as recording electrodes.
In SSR analysis rapid development of habituation after consecutive electrical stimulations is a well-known rule [9]. Therefore, electrical stimulation was applied to the contralateral median nerve in total 4 times with at least a 3-minute interval, thereby avoiding habituation. The average of these four stimulations was calculated. During recording the filter setting was between 0.5 Hz and 2 kHz, stimulation time was 0.01 s and the stimulation level was 25 mA. SSR latency was measured by taking the negative deflection starting point into account, whereas amplitude of SSR was measured by taking peak points of both negative and positive deflections into account.
Electrocardiographic study
In all patients ECG analysis was carried out before and 1 month after treatment. Heart rate, P-wave time, PR distance, PR segment, QRS time, T-wave time, QT distance, QTc distance, T peak-end (Tpe), T peak-end/QT (Tpe/QT), and T peak-end/QTc (Tpe/QTc) parameters were observed.
Results
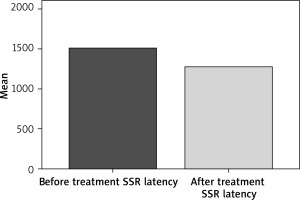
In patients SSR analysis was carried out before and 1 month after treatment. Prior to treatment average SSR latency was 1506.43 ±246.20 ms, whereas SSR latency average after treatment was 1275.03 ±170.71. The SSR latencies after treatment were obviously shorter than those before treatment and the difference was statistically significant (p < 0.001) (Figure 1).
Before treatment the average SSR amplitude was 2.69 ±1.68 mV. After treatment the average SSR amplitude was 2.27 ±1.80 mV. The SSR amplitude after treatment was slightly lower, but the difference was not statistically significant (p = 0.365).
In patients ECG scanning was performed before and 1 month after treatment. Before treatment the heart rate was 85.21 ±15.59 beats/min, but after treatment it was 81.07 ±15.63 beats/min. No significant difference was found when QTc distance, Tpe, Tpe/QTc values and other ECG parameters, which are predictors of pre-treatment malign cardiac arrhythmia, were compared with the values obtained after treatment. All ECG parameters are shown in detail in Table 1. Moreover, pre-treatment systolic blood pressure was 111.78 ±9.83 mm Hg, but it was 112.77 ±10.40 mm Hg after treatment. Pre-treatment diastolic blood pressure was 68.21 ±7.72 mm Hg, but it was 70.92 ±8.55 mm Hg after treatment. No significant difference was found in the blood pressure values before and after treatment (p > 0.05).
Table 1
ECG parameters
Discussion
Acne vulgaris is a chronic inflammatory disease of the pilosebaceous unit, and it occurs in skin regions where the most sebaceous glands exist. In 99% of patients it affects the face [10]. Although it predominantly affects the adolescent population (approximately 85%), pre- and post-adolescents can also be affected [11, 12]. Although there are several treatment options for acne vulgaris, only isotretinoin has an influence with effects on all primary etiological factors [13]. But in most of the patients an increase is observed in acne lesions for the first month [14].
Until now there has been no study researching the effects of isotretinoin (used in the treatment of acne vulgaris) in the autonomic nervous system by carrying out SSR analysis. In this study we aimed to reveal both negative and positive effects of isotretinoin in acne vulgaris treatment by examining the effects of the medication in autonomic and cardiac functions apart from the potential effects of it by performing SSR analysis and ECG scanning in subjects.
Skin is innervated with sensory nerves, postganglionic sympathetic and parasympathetic nerve fibers. In general, it is accepted that sebaceous glands are not innervated by the peripheral nervous system. However, it was reported that the number of nerve fibers around sebaceous glands in facial skin is high in acne vulgaris patients, whereas it was found that nerve fibers in normal facial skin were rare [15].
In a study that included 50 subjects with atopic dermatitis and 50 healthy subjects conducted by Wruhs et al., sudomotor activity (activity of sweat glands) in the two groups was measured by the SSR method [15]. It was found that the average SSR latency was significantly longer in the atopic group than the control group. Average SSR amplitude was lower in the atopic group than the control group, but the difference was not statistically significant. Wruhs et al. postulated that extension of SSR latency may be an indication of sympathetic innervation deficiency [15]. The findings of Wruhs et al. suggest that, as in dermatomyositis or polymyositis, the nerve fibers within the related tissue are secondarily affected by inflammation. In parallel with the results of Wruhs et al., in our study pre-treatment SSR latencies were notably and statistically significantly longer than those after treatment. This situation recalls a sympathetic dysfunction in both atopic dermatitis and acne vulgaris.
Kaplan et al. observed that in patients with acne vulgaris for which thoracoscopic sympathectomy was performed, acne lesions remitted after sympathectomy [16]. During isotretinoin treatment, at the end of the first month of treatment, an increase in sympathetic activity can explain the exacerbation of acne lesions during the first month in most of the patients. However, when it is considered that normal isotretinoin treatment can take 20 weeks, if the SSR study had continued in months 2, 3, 4 and 5, probably SSR latencies would have been longer in the following months when compared to the first month. Therefore, we can assume that there was a role of isotretinoin in the sympathetic activity decrease for remission of acne lesions. Unfortunately, we could not achieve sufficient cooperation with the patients included in the study for a long-term study. Therefore, the study was planned as before treatment and 1 month after treatment.
The increase in sympathetic activity during the first month of isotretinoin treatment can be explained as follows. SSR is known to include suprasegmental inputs from the cerebral cortex and inhibitory inputs from the striatum and to reflect the activity of the posterior hypothalamus and brainstem reticular formation [17]. From this point of view, it can be surmised that by depressing the inhibitory inputs within isotretinoin striatum, suprasegmental structures, partially released, could increase the sympathetic activity just the same as in the increase of the deep tendon reflex in first motor neuron disease after elimination of inhibitory mechanisms. Another mechanism of increase in sympathetic activity by isotretinoin is that it can cause an increase in nerve growth factor (NGF). Nerve growth factor is the best categorized member of the neurotrophin family; it is vital for survival of peripheral sympathetic and sensory neurons and basal frontal cholinergic neurons within the central nervous system [18].
In our study, before treatment SSR amplitude average was 2.69 ±1.68 mV and after treatment SSR amplitude average was 2.27 ±1.80 mV. After treatment SSR amplitudes were slightly lower compared to before treatment, but there was no significance in values statistically. When it is considered that isotretinoin causes apoptosis of sebaceous glands, it can be expected that we obtain a SSR response with lower amplitude than sebaceous glands atrophied by isotretinoin just the same as M and N response obtained from motor and sensory fibers of atrophied tissue in routine electrophysiological studies.
In 1992 Drory et al. classified 100 healthy subjects based on the supposition that electrophysiological differences may occur depending on age, and they carried out an SSR analysis and observed that latency values extend with aging, amplitude values decrease, or a response can be obtained. Nevertheless, it is not known which factors decrease or increase the amplitude values. It is beyond doubt that there is a need for further research on this topic [19]. The average age of our patients was 20.93 ±5.03.
Although the effects of isotretinoin on many systems are well known, its effects on the cardiovascular system have rarely been reported. Some parameters in ECG can be used to predict potential arrhythmias. Yan et al. indicated that parameters such as Tpe time, Tpe/QT time, and Tpe/QTc can be used to predict ventricular arrhythmia formation [20]. In a study conducted in 45 patients receiving isotretinoin treatment for 6 months due to acne vulgaris, Dursun et al. observed no difference in QTc and QT dispersion, which is an indicator of malign ventricular arrhythmia, and in these patients it was safe in terms of ventricular arrhythmia for 6 months of acne treatment [21]. Although rare, in the literature there are cases indicating that isotretinoin can cause ventricular extrasystole and atrial tachycardia attacks [22, 23].
In our study, P-wave time, PR distance, QRS wave time, QT time, QTc time, Tpe time, Tpe/QT time, and Tpe/QTc time of patients were assessed with ECG scanning before and one month after isotretinoin treatment. Moreover, patients were monitored for tension before and after treatment. It was observed that there was no significant change in basal ECG parameters and Tpe, Tpe/QT and Tpe/QTc times, which may be an indicator of malign ventricular arrhythmia before and after treatment. In our study, during one-month isotretinoin treatment no cardiac arrhythmia or electrocardiographic changes were observed in patients.
Conclusions
Quantitative changes in SSR and ECG parameters were measured in patients using isotretinoin due to acne vulgaris. As a result, this study showed that SSR results indicating that isotretinoin increases the existing sympathetic activity in acne vulgaris can explain the exacerbation in acne lesions for the first month and according to the ECG results the medication shows no cardiac side effects; therefore, this study is quite important.