Introduction
Hirsutism, a common complaint in women visiting a dermatological department, refers to abnormal growth of the terminal hair in the male-like pattern. It may be a result of androgen excess or an increased sensitivity of the hair follicle to normal levels of androgens [1]. Many laboratory markers or clinical signs are used in the evaluation of androgen production. The most often reported parameter is the level of total testosterone (TT) or calculated free testosterone (cFT) [2].
The modified Ferriman-Gallwey (mFG) score has now become the gold standard for determining the density of terminal hairs at nine different body sites (i.e. upper lip, chin, chest, upper back, lower back, upper abdomen, lower abdomen, arms and thighs); a total score of 8 is considered as hirsutism and was chosen as a clinical marker of hyperandrogenism [3]. The polycystic ovarian syndrome (PCOS) is the most frequent cause of hirsutism in this population, and the prevalence of the syndrome increases throughout puberty [4].
Many hirsute women are found to have increased circulating androgen levels, although, in some of them, androgen levels are within normal range [3, 5].
Some studies show that the severity of hirsutism correlates poorly with the severity of androgen excess; it has not yet been clarified which of the androgenic parameters has the most significant relationship with the mFG score [3, 6].
Although androgens modulate the biological mechanism regulating the hair cycle, limited studies have evaluated the relationship between clinical and biochemical hyperandrogenemia and these have reported conflicting findings.
The variances in published results indicate that hyperandrogenism not only reflects circulating androgen levels but is also influenced by the peripheral metabolism of androgens [7].
Aim
We aimed to investigate the association between biochemical hyperandrogenism and mFG score for better understanding of the pathophysiology of excess hair in women; this information will potentially be helpful for selection of treatment options.
Material and methods
Setting
This was a cross-sectional study of 300 premenopausal Iraqi hirsute women. They were referred to Faiha Specialized Diabetes, Endocrine, and Metabolism Center (FDEMC) in Basrah (Southern Iraq) in the period between August 2016 and August 2017.
Research ethics approval was obtained from the Ethics Committee of Basrah University before the initiation of the study, and signed informed consent was obtained from all included patients. None of the patients suffered from any chronic or acute diseases. Women who were pregnant or lactating, those who received oral contraceptive pills or other drugs that could interfere with the hormonal markers, the premenarchal and postmenopausal women were excluded from the study.
Definitions
The degree of hirsutism was assessed using mFG score in the face (chin, upper lip), chest, upper back, lower back, upper abdomen, lower abdomen, thighs, and upper arms in addition to the sideburns, lower jaw/neck and buttocks and perineum [1]. In each of these areas, a score of 0 (absence of terminal hairs) through 4 (extensive terminal hair growth) was assigned. Total scores were obtained for each woman. The maximum score was 36. A score of eight or more was considered as hirsutism according to the mFG scoring system [8].
Hirsutism was considered as mild (score 8–16), moderate (score 17–24), and severe (score > 24) [9]. A single examiner performed the scoring assessment on each patient. In addition to the FG scoring, the height and weight were recorded by a stadiometer (SECA –763)TM. Body mass index (BMI) was calculated by using a formula: weight (kg) divided by the square of height (m2).
Blood sampling
The blood samples were collected between 8:00 and 9:00 a.m. following 8–10 h of fasting. Fasting venous blood specimens were collected in tubes containing the clot activator. The sampling was taken in the early follicular phase of the spontaneous or progesterone induced menstrual cycle (day 3–5) [10].
The serum separator tube specimens were allowed to clot and were then centrifuged for 10 min at 3000 × g to separate the serum. In all cases, serum TT, dehydroepiandrosterone sulfate (DHEA-S), sex hormone binding globulin (SHBG) were measured on the same day using fully automatic chemiluminescence immunoassay kits Cobas e411 analyzer series (Roche Diagnostics).
FT was calculated from TT and SHBG using an online based calculator (http://www.issam.ch/freetesto.htm).
Hyperandrogenemia was defined as a cut-off value of TT ≥ 57.7 ng/dl (2.0 nmol/l), FT > 1.9 ng/dl (26 pmol/l) and DHEA-S > 368 μg/dl (10 μmol/l) [11].
Statistical analysis
Data of the 300 hirsute patients were analysed using the statistical package for social science (SPSS), version 24. Data were checked for errors, inconsistency, and outliers using data cleaning methods. Descriptive statistics presented as mean, standard deviation or standard error for the mean, frequencies and proportions (%).
Analysis of variances was utilized to compare means (more than two), for ensuring mean body area scores, or mean hormonal levels across the severity of hirsutism. The level of significance of ≤ 0.05 was considered a significant difference or correlation. Results and findings were shown in tables and figures with an explanatory paragraph to each corresponding one using the Microsoft Word software for Windows, version 2016.
Results
Clinical and hormonal parameters are summarized in Table 1. According to mFG scoring system with the use of Abraham’s classification, the patient’s frequency was as follows: there were 18 (6.0%) mild cases, 116 (38.6%) moderate cases and 166 (55.3%) severe cases. Only 59 (19.7%) patients had a normal BMI, 84 (28.0%) patients were overweight, and 157 (52.3%) patients were obese. The mean levels of androgen among the participants were as following: TT 39.37 ±24.96 ng/dl, SHBG 45.67 ±38.87 nmol/l, FT 0.84 ±0.70 ng/dl, DHEA-S 306.11 ±186.43 μg/dl.
Table 1
Clinical and laboratory characteristics of patients
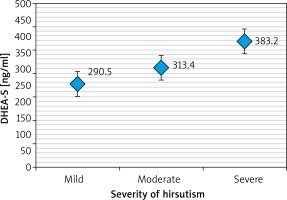
Table 2 revealed that a high TT level was significantly more frequent in patients with severe hirsutism compared to those with mild or moderate hirsutism (p = 0.047). Another significant correlation was found between higher FT levels and severe hirsutism (p < 0.001) while there was no significant correlation found between the severity of hirsutism and DHEA-S level (p > 0.05).
Table 2
Relationship between severity of hirsutism and levels of dehydroepiandrosterone sulphate (DHEA-S), total testosterone (TT) and free testosterone (FT)
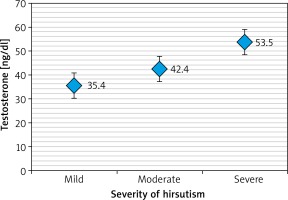
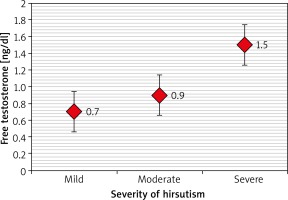
Table 3 and Figures 1–3 revealed that patients with severe hirsutism had significantly higher mean levels of TT and FT compared to those with mild and moderate hirsutism (p < 0.05), while no significant difference had been found in mean DHEA-S across the severity of hirsutism (p > 0.05).
Table 3
Comparison of mean levels of total testosterone (TT), free testosterone (FT), and dehydroepiandrosterone sulfate (DHEA-S) according to the hirsutism severity
Discussion
While various hormones have been linked with hirsutism, it is unclear which serum test reflects the clinical situation most accurately and best correlates with the degree of hirsutism [12]. Testosterone is perhaps the most commonly studied androgen in hirsute patients, but not all hirsute women have clear evidence of detectable serum testosterone or other androgen excess using routine laboratory methods.
Bias can potentially be introduced by patients or the individuals conducting the assessment. Even knowledge of laboratory results can be associated with ascertainment bias. Our present study combated this bias by having one trained examiner assessing each patient before gaining knowledge of laboratory results.
Testosterone circulates in plasma non-specifically bound to albumin and specifically bound to SHBG; a small fraction is unbound as free testosterone. Testosterone is subject to variation in time and direct and indirect hormonal changes influencing the menstrual cycle.
As androgens play a definite role in the transformation of vellus into terminal hair during puberty, and in the growth of terminal hair by prolonging its androgen phase in the androgen-dependent areas of the female body, hirsutism is considered as a clinical marker of androgen excess [13].
We found that there was a definite correlation in the severity of hirsutism with the significant impact of the FT level and, to a lesser extent, to TT. Moreover, we demonstrated a negative correlation between hirsutism score and the serum DHEA-S level, which led us to speculate that the severity of hirsutism mainly depends on bioavailable circulating and FT.
For severity of hirsutism, there were stronger associations with FT rather than TT. This means that it will be of a greater benefit to use FT as a better predictor for evaluating androgen status. Measurements of FT is 50% more sensitive than that for TT in detecting androgen excess and is the best single indicator of hyperandrogenism. Hirsutism has been correlated with increased levels of serum androgens. FT has been reported to have a stronger correlation with a clinical diagnosis [14, 15].
The measurement of circulating DHEA-S in hirsute women is of unclear value and might not reflect the status of steroidogenesis. Additionally, DHEA-S has a little or no capacity to bind to the androgen receptors and require conversion to testosterone to exert androgenic effects. DHEA-S accounts for only 5% of circulating androgens among women of reproductive age. A few studies demonstrate that the severity of hirsutism correlates inadequately with the severity of androgen excess; it has not been explained until now which of the androgenic parameters have the most relevant association with FG score [16, 17].
In other studies, authors reported that for hirsutism and PCOS, obese patients are particularly associated with increased FT [3, 6]. Another study by Hahn et al. reported a significant correlation of androgen indices with hirsutism score, ovarian volume and follicles count [18].
Many hirsute women have been found to have increased circulating androgen levels; however some of them have normal androgen levels [3, 5, 6]. Interestingly, the distinct pattern of relationships among FT, TT and DHEA-S levels across affected and unaffected body areas were completely different.
It has long been recognized that the degree of hirsutism is affected by several factors including local androgen concentrations at the pilosebaceous unit, increased levels of androgens [2], defects in 5α-reductase production, androgen receptor activity and other key enzymes [19]. Moreover, the severity of hirsutism can differ by age [20], race, ethnicity and familial background [20, 21].
We did not measure DHT and androstenedione, which are the important potent androgens and best ones to identify the impact of androgens on hair follicles.
Conclusions
The FT correlated more than TT with the severity of hirsutism using the mFG scoring system. The level of DHEA-S does not correlate with the severity. A more extensive study is needed to assess the clinical relevance of mFG scoring and population specific diagnostic cut-off scores for hirsutism.