Introduction
Asthma is a heterogeneous group of diseases involving chronic inflammation of the respiratory tract. One characteristic feature of asthma is bronchial hyper-reactivity, i.e. variable and reversible air flow restriction in the airways, which occurs spontaneously or in response to various physical, chemical, or biological stimuli. Chronic airway inflammation produces clinical manifestations and, over time, also tissue remodelling, which leads to respiratory dysfunction. Characteristic clinical manifestations of asthma include paroxysmal dyspnoea, cough, chest tightness, and episodes of wheezing. The diagnosis is based mainly on the clinical presentation and lung function test results [1–3]. Asthma is the most common chronic disease in children and one of the most common chronic diseases in adults. Thus, asthma unquestionably constitutes a serious health problem as it affects up to 339 million people around the world, and its prevalence continues to rise [4]. Surveys conducted in Poland in the years 2006–2008 indicated asthma in 4.6% of respondents, with 10.6% of the respondents diagnosed with asthma based on a clinical examination [5]. Asthma is a multifactorial disease, with the risk factors including certain individual characteristics and environmental factors. The known individual characteristics include: genetic predisposition, sex, atopy, comorbid allergies, obesity, and prenatal risk factors. Well-established environmental factors include exposure to inhaled allergens, respiratory infections, active and passive exposure to cigarette smoke, air pollution, exhaust fumes, and diet [1–3]. Asthma is a chronic inflammatory condition, often beginning in childhood and persisting throughout the patient’s life. Therefore, the prophylactic, educational, and therapeutic measures that would help control the symptoms of asthma and allow the patient to develop normally and maintain everyday activities and a high quality of life are of crucial importance. According to the current position of the Global Initiative for Asthma (GINA) [1], asthma control assessment is based on two aspects: the control of symptoms and assessment of the risk of future adverse outcomes defined as disease exacerbation, sustained airflow obstruction, and drug-related side effects.
Aim
The purpose of this study was to assess asthma-associated symptoms with use of a questionnaire in an attempt to identify further measures intended to improve asthma control. Due to the chronic nature of obstructive respiratory changes in asthma, such phrasing of the study purpose seems to be particularly rational in light of the sheer scale of the phenomenon of this disease, particularly in this difficult era of the pandemic. Identifying and introducing appropriate systemic solutions undoubtedly also ensure adequate disease control from the point of view of the individual and of the society.
Material and methods
This study was conducted via a computer-assisted personal interviewing (CAPI) technique with the use of a personal digital assistant (PDA), a tool commonly referred to as a palmtop. The study population had been randomly selected from the Ministry of the Interior and Administration database of Polish resident identification numbers (PESEL numbers) and is an extension of the European Community Respiratory Health Survey II (ECRHS II) [6] and International Study of Asthma and Allergy in Childhood (ISAAC) [7]. In its design, this study included a study population from eight of Poland’s largest cities (Gdansk, Wroclaw, Poznan, Katowice, Krakow, Lublin, Bialystok, and Warsaw) and one rural area (Krasnostawski County). These areas had been selected based on the ECRHS criteria of approximately [6] 150,000 inhabitants, areas enclosed within the existing administrative boundaries, with an up-to-date sampling frame from which a sample of 20–44-year-old people can be selected. The respondents were selected randomly in each study centre. The study consisted of two stages, with 22,500 respondents included into the first stage based on their responses in the questionnaire; the second stage involved an outpatient examination and included 7,000 respondents, who underwent skin-prick testing for the allergens of birch, grasses/cereals, Dermatophagoides pteronyssinus and Dermatophagoides farinae, type-I moulds (Botrytis cinerea, Cladosporium herbarium, Alternaria tenuis, Curvularia lunata, Fusarium moniliforme, Helminthosporium), type-II moulds (Aspergillus fumigatus, Mucor mucedo, Penicillium notatum, Pullularia pullulans, Rhizopus nigricans, Serpula lacrymans), cat, dog, a control solution, histamine). Allergic rhinitis was diagnosed based on the Allergic Rhinitis and its Impact on Asthma (ARIA) initiative criteria, and asthma was diagnosed based on the GINA criteria.
The study population comprised 18,874 respondents, including 4,621 children aged 6–7 years, 4,832 adolescents aged 13–14 years, and 9,421 adults aged 20–44 years. An overwhelming majority of the respondents (n = 16,482) were urban residents, and only 2,392 of respondents resided in rural areas (Table 1).
Table 1
Study population (stage I: questionnaire-based study)
Statistical analysis
Statistical analysis included contingency tables, proportions of individual allergic conditions, rates of their symptoms, and the rates of allergies to selected allergens. The study had been approved by the Ethics Committee of the Medical University of Warsaw (KB/206/2005) and the Inspector General for the Protection of Personal Data.
Results
The prevalence of declared allergic rhinitis (considering the age criterion) ranged from 34.6% to 37.9% of the respondents. Allergic rhinitis was decidedly more commonly reported by urban residents than by rural residents (with no differences in distribution between the two sexes). Seasonal allergic rhinitis was diagnosed in 14.7% of all respondents, predominantly in the 20–44-year-olds. Conversely, perennial allergic rhinitis was predominant in adult males from urban areas. Asthma was declared by 12.2–19.4% of all respondents, and was most common in males and nearly twice more common in urban residents than in rural residents. The cases of asthma in the study population were mostly moderate in severity, and asthma predominantly affected 13–14-year-old adolescents, most commonly boys from urban areas (Table 2).
Table 2
Study population (stage II: outpatient assessments)
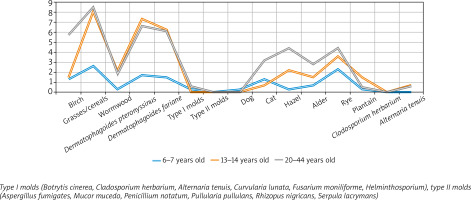
Allergic rhinitis and asthma co-occurred in 523 (11.6%) of the evaluated 6–7-year-olds, 311 (6.6%) of the evaluated 13–14-year-olds, and in 723 (7.7%) of the evaluated adults. Comorbid allergic rhinitis and asthma were most common in males (779 (9.1%) cases in males vs. 778 (7.8%) cases in females) and relatively more common in urban than rural residents (1,445 (8.7%) cases vs. 112 (5.5%) cases, respectively). The rates of positive skin-prick tests were based on wheal diameters of > 9 mm. The allergens producing the greatest number of pronounced positive responses to topical allergen application (in the asthma subgroup) in the order of decreasing significance were as follows: grasses/cereals, Dermatophagoides pteronyssinus and Dermatophagoides farinae, hazel, and rye. Allergies to grasses/cereals were significantly more common in urban residents than in rural residents (6.6% vs. 1.4%, respectively). Interestingly, no significant differences in skin-prick responses were observed between the two sexes. The study participants showing an allergy to house dust mites were predominantly adult males (6.7% of men vs. 2.9% of women) and residents of urban areas (5.0% of urban residents vs. 2.7% of rural residents). Comparably to the sex distribution of house dust mite allergies, the allergies to hazel and rye were decidedly most common in men than in women (3.5% vs. 13%, respectively, for hazel; 4.0% vs. 2.9%, respectively, for rye) who resided in urban areas (Figure 1).
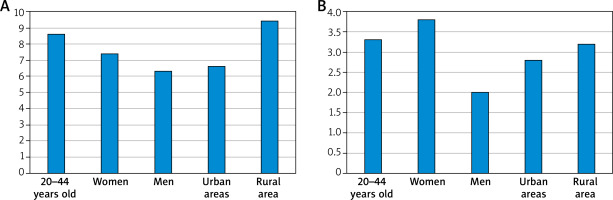
A recent asthma attack was declared mainly by females from urban areas. Symptoms such as periodic difficulty breathing were reported by nearly 90.1% of children aged 6–7 years, 89.6% of adolescents aged 13–14 years, and 80.7% of the evaluated adults. A relatively low proportion (9.9%–19.3%) of the total study population reported breathing difficulties that were chronic. In their answer to the question How many times a week on average have you been woken by shortness of breath in the last 3 months? the respondents declared that their sleep was interrupted by an asthma attack over 3 times a week (specifically: 3.6 for adults, 3.6 for women, 3.1 for men, 3.3 for urban residents, and 4.6 for rural residents). The mean age at the most recent asthma attack was declared as 26.2 years in 20–44-year-olds; 18.4 years in females; 14.5 years in males; 16.4 years in urban areas, and 15.03 years in rural areas). Inhaled steroid therapy was introduced in the group of adults at the age of approximately 25 years (12.6 years in women, 9 years in men, 10.6 years in urban residents and 7.7 years in rural residents), and the mean treatment duration was 4 years (4.5 years in the group of 20–44-year-olds, 3.6 years in women, 3.4 years in men, 3.4 in urban residents, and 4.3 years in rural residents) (Figure 2).
Discussion
One very important aspect of evaluating asthma control is symptom severity assessment. Such symptoms as wheezing, cough, or tightness in the chest vary in severity, and experiencing them is frequently associated with the risk of disease exacerbation. The experts behind the GINA recommendations emphasize the importance of asking patients direct questions about their symptoms at every visit [1]. Questionnaires and numerical rating scales help rate symptom severity. Such tools include the 7-item Asthma Control Questionnaire (ACQ) that yields scores of 0–6 points and the 5-item Asthma Control Test (ACT), with scores ranging from 0 to 25 points. Another important component of controlling asthma is conducting lung function tests. Although the results of function tests do not always correlate with the severity of asthma symptoms, they are a useful tool in treatment follow-up. Another very important aspect in asthma control is the response to treatment. The goal of treating asthma is to control the disease and prevent its exacerbations and long-term complications [8].
Recent years have seen a continual increase in the incidence of allergic conditions, including asthma. According to the 2018 Global Asthma Report, there are 339.4 million people with asthma worldwide. In Europe, the number of affected individuals is approximately 30 million. According to GINA data, the proportion of asthma patients in the general population varies widely and ranges from 1% to 18%. Such considerable differences in the results of epidemiological studies are most likely due to the complex definition of the disease and the difficulties in its diagnosis [1, 4]. In China there are 45.7 million people (4.2%) affected with asthma, whereas in Saudi Arabia the number of asthma patients is approximately 2 million, which is 6% of the country’s general population [8, 9]. In our study, which evaluated a population of 18,617 people, asthma was diagnosed in 2,508 (13.4%) people, including 1,272 (12.7%) women and 1,236 (14.4%) men. According to the current version of the GINA recommendations, disease control assessment is one of the key aspects of care in people with asthma. Disease control assessment is based on assessing the current symptoms and predicting future adverse phenomena, such as disease exacerbation, sustained respiratory air flow obstruction, and drug-related side effects [1]. The assessment of asthma control in our study showed the disease to be well controlled in 76 (2.9%) women and in 90 (4.1%) men. Cases of well-controlled asthma were observed in all age groups (in 2.8% of adults, in 3.8% of adolescents aged 13–14 years, and in 4.3% of children aged 6–7 years). A 24-week multi-centre prospective randomized study by Ye et al. compared the efficacy of treating asthma with and without the use of ACTs in a Chinese population. Those authors reported a considerably greater effectiveness of treatment and care in the group that used an ACT [8]. Moreover, randomized Italian and Japanese studies show the usefulness of ACTs in monitoring asthma and the effectiveness of its treatment and emphasize good tolerance and acceptance of this disease control tool by patients [10, 11]. In recent years, the issue of multimorbidity – or the co-existence of one or more diseases with the disease of interest – and the associated multidisciplinary multi-specialist patient care have been areas of particular interest in the field of allergy and other branches of medicine. In 2015, Bousquet et al. first described the phenomenon of multimorbidity among allergic conditions: allergic rhinitis, asthma, and atopic dermatitis, paying particular attention to their similar immune and non-immune pathophysiology [12]. Cingi et al. presented the phenomenon of multimorbidity with respect to allergic rhinitis. Those authors proposed a classification of allergic rhinitis comorbidities into: (a) allergic conditions (asthma, atopic dermatitis, food allergies, anaphylaxis); (b) conditions affecting organs located near the nose (otitis media, sinusitis, conjunctivitis), (c) problems with sleep and concentration; and (d) turbinate hypertrophy [13]. Our study revealed the coexistence of allergic rhinitis and asthma. These conditions coexisted in 11.6% of the evaluated 6–7-year-olds, in 6.6% of the evaluated 13–14-year-olds, and in 7.7% of the evaluated adults. The coexistence of allergic rhinitis and asthma was shown to be more common in men than in women (9.1% vs. 7.85%), particularly in men living in urban areas. The nose and paranasal sinuses are an integral part of the respiratory tract. Anywhere between 19% and 38% of patients with allergic rhinitis also have asthma, and most patients with asthma also have allergic rhinitis. This observation gave rise to the “united airway disease” concept [14–16]. A study by Togias demonstrated that 85–95% of patients with asthma also have allergic rhinitis. Moreover, the presence of allergic rhinitis exacerbates the symptoms of asthma, increases the rate of exacerbations, and considerably worsens asthma control [16]. A study by Morjaria et al. also demonstrated that effective treatment of allergic rhinitis, particularly via allergen-specific therapy, prevents the development of asthma [17]. De Vittori et al. assessed the proportion of allergic rhinitis in children with asthma and the effects of allergic rhinitis on asthma control [18]. The study evaluated 60 children diagnosed with asthma at the age of 5–11 years and also allergic to house dust mites. Eighty percent of those children were shown to have rhinitis. The study demonstrated that nasal obstruction due to allergic rhinitis worsened asthma control [17].
One very interesting paper by Clark emphasizes the fact that some of the extremely important aspects of asthma treatment and control, apart from clinical care, are the cooperation between the parents of asthmatic children and the school authorities, as well as development of novel educational programs supporting this process [19].
Additionally, as we have pointed out elsewhere, effective and efficient management of the diagnostic and therapeutic processes in the care of patients with chronic allergic conditions may be implemented as part of the coordinated health care concept [20]. In order to introduce this in practice in patients with asthma, we should start from primary health care to define the area as well as components of coordinated health care for patients suffering from chronic allergies as we showed our proposal of the areas and components in the framework of coordinating health care for a patient suffering from chronic allergies in Poland [20]. Subsequently, we should start to include outpatient specialist care and in-hospital care into coordinated health care for patients suffering from chronic allergies.
We would also like to emphasize that the main process, i.e. the process of care for patients with allergic conditions, is complemented by management-related processes, including the management of information in the form of new data from the monitoring of the condition of allergic patients. The database system containing information obtained from patient monitoring provides data that can be used by a health care professional, i.e. a physician. The expert systems used in the management of allergic conditions via chronic condition assessment may support the entire health care system in caring for the patient, via telediagnostics and continual disease monitoring via the internet. This additional, systemic, control in the process of independent management will increase therapeutic effectiveness by ensuring regular follow-up visits through increasing the patient’s awareness of health care system organization [21]. Thus, as part of organizational solutions adopted in Poland for the treatment and control of asthma, apart from regular, systematic, long-term clinical care, it is important to establish cooperation with:
– patients with asthma – irrespective of their age,
– parents, guardians or parents of children with asthma,
– school authorities (teachers, and especially the team of educators and psychologists), and
– collective development of novel educational programmes, with the use of e-learning methods (targeting children, parents, teachers, and health care professionals) as a means of supporting the process of care for chronically ill patients, also in the difficult times of the pandemic.
This is a very important aspect of integrated care for asthma patients and it seems to be particularly valid and needed during the COVID-19 pandemic, when telemedicine and remote disease control have become so important.
Conclusions
As a group of heterogeneous conditions with an underlying chronic inflammation of the respiratory tract, asthma requires special prevention and treatment measures realized systemically at every level of integrated and coordinated health care for chronically ill patients, in order to minimize the risk of complications. A good (that is integrated and coordinated) health care for chronically ill patients with asthma should be provided:
– systematically, i.e. based on the appropriate number of follow-up examinations, planned based on clinical standards; symptom monitoring, and scheduled doctor’s visits,
– appropriately, i.e. be adjusted to the asthma patient’s age and needs,
– and based on established clinical and organizational standards. Asthma control ensures not only functional independence on the individual level, but also is of great social benefit reducing the burden of bronchial obstruction in the society.