Introduction
Hepatocellular carcinoma (HCC) was the third most common cause of cancer-related deaths globally in 2020 and the sixth most prevalent cancer overall [1]. Despite significant improvements in the monitoring, diagnosis, and care of HCC patients, most cases are unfortunately detected at an advanced stage with a dismal prognosis [2].
Systemic treatment has advanced considerably in the last decade for HCC patients unsuitable for locoregional therapy [3]. Since being approved in 2007 [4], sorafenib (SOR), a multi-kinase inhibitor (MKI), has been routinely utilized to treat advanced HCC. Lenvatinib (LEN), another oral MKI, was approved in 2018 as first-line therapy for unresectable HCC patients after the REFLECT study [5]. It has multiple targets, including fibroblast growth factor (FGF) receptors 1-4, RET, KIT, platelet-derived growth factor (PDGF) receptor α, and vascular endothelial growth factor (VEGF) receptors 1-3. In a recent meta-analysis, LEN was reported to have better progression-free survival (PFS) (hazard ratio [HR] = 0.63, 95% confidence interval [95% CI]: 0.53-0.74), objective response rate (ORR) (odds ratio [OR] = 5.61, 95% CI: 3.90-8.09), and disease control rate (DCR) (OR = 2.42, 95% CI: 1.79-3.28) compared to SOR [6].
Subsequently, immune checkpoint inhibitors (ICI) were introduced for the treatment of HCC. The cornerstones of ICI are programmed death receptor 1 (PD1) and anti-programmed cell death ligand 1 (PDL1) inhibitors. Combining antiangiogenic drugs increases the effectiveness of ICI through vessel normalization and tumor sensitization to antiangiogenic therapy by ICI [7]. Atezolizumab (anti-PDL1) and bevacizumab (anti-VEGF) together significantly improved the median PFS (6.8 vs. 4.3 months, p < 0.001) and OS (13.2 months vs. not reached, p < 0.001) when compared to SOR, as revealed in the IMbrave 150 trial [8]. Currently, atezolizumab with bevacizumab (ATE/BEV) is the first-line treatment of choice in advanced HCC in addition to LENV/SOR/durvalumab [9].
ATE/BEV combination has not been evaluated head-to-head with LEN in randomized trials. Hence, we conducted this meta-analysis to compare the therapeutic efficacy, clinical outcomes, and safety of ATE/BEV and LEN as first-line treatments for advanced HCC (aHCC) patients.
Material and methods
Information sources and search strategy
A comprehensive search of all suitable studies was conducted using the databases of MEDLINE, EMBASE, and Scopus from inception to December 2022. The keywords used were: (Atezolizumab AND Bevacizumab AND Lenvatinib) AND (HCC OR ‘Hepatocellular carcinoma’ OR ‘Hepatic carcinoma’ OR ‘Liver cancer’). To ensure that no potentially relevant items were overlooked, manual searching of reference lists of the included studies was also undertaken. The study methodology was designed and executed to adhere to the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines [10].
Study selection
The PICO criteria used for included comparative studies were: a) Population – aHCC; b) Intervention – ATE/BEV for aHCC; c) Comparison – LEN for aHCC; d) Outcomes – treatment response, overall survival (OS), PFS, and adverse events (AE). The treatment response was based on the modified RECIST criteria [11]. Objective response rate was defined as the percentage of patients with partial or complete response. Disease control rate was defined as the percentage of patients with a response or stable disease. Following the selection criteria above, the titles and abstracts of all studies were independently reviewed by two authors. A third reviewer resolved any disagreements. The exclusion criteria used were: non-comparative studies, case series, and studies involving persons < 18 years of age.
Data extraction
Two independent reviewers performed the data extraction, and a third reviewer resolved any disagreement. Data were collected under the following headings: study author and year, country of study, study design, number of patients, age and sex distribution, details of liver disease, BCLC staging, follow-up, and outcomes.
Risk of bias in individual studies
After data extraction, the same two reviewers performed a quality assessment using validated tools. The Newcastle-Ottawa scale for cohort studies was used for the quality assessment of included studies. NOS scores of 1-4, 5-7, and 8-9 were considered low, intermediate, and high quality, respectively.
Statistical analysis
Results were presented with their 95% CI as the HR for time-to-event outcomes or OR for dichotomous outcomes. Regardless of heterogeneity, the Mantel-Haenszel test for random effects was used. Cochran’s Q test and I2 statistics were used to determine the heterogeneity between the studies. A p-value of the Q test < 0.1 or the I2 value > 50% was considered significant. Any potential publication bias was verified through a visual assessment of funnel plots. The sensitivity analysis was performed using a leave-one-out meta-analysis. One study is excluded at each analysis to analyze each study’s influence on the overall effect-size estimate and identify influential studies. RevMan software (version 5.4.1, Cochrane Collaboration) and STATA software (version 17, StataCorp., College Station, TX) were used for statistical analysis.
Results
Study characteristics and quality assessment
The search strategy yielded 1142 records, of which 784 were screened after removing duplicates. Finally, eight studies [12-19] were included in the meta-analysis. Figure 1 shows the PRISMA flowchart for the study inclusion process. Tables 1 and 2 show the baseline characteristics and outcomes of included studies, respectively. The majority of studies were from Japan and Europe, with sample sizes varying from 92 to 2205. Only the study by Maesaka et al. was prospective [14]. The median age of the patients varied from 62 to 76 years. Viral etiology was the commonest in all studies. The majority of patients belonged to the BCLC-C category. Extrahepatic metastasis was present in 26.3% to 62.3% of cases. The follow-up duration varied from 7.2 to 18 months, with a longer follow-up duration in the LEN groups in the majority of the studies. On quality assessment, all but two studies [13, 18] were of good quality, as shown in Table 3.
Table 1
Baseline characteristics of the included studies
| Author | Country/ Study design | Drug | No. of patients | Age (years) | Male/ female | Viral etiology n(%) | BCLC B/C | MVI/EHM n(%) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Hiraoka | Japan, | ATE/BEV | 194 | 74 (68-79) | 148/46 | 102 | 93/101 | 44 (22.7)/71 (36.6) | 8.0 (4.9-11.2) |
| et al.2023 [13] | retrospective* | LEN | 57 | 73 (69-79) | 41/16 | 27 | 34/23 | 5 (8.8)/15 (26.3) | 14.4 (9.3-19.0) |
| Kim et al. 2022 [14] | Korea, retrospective* | ATE/BEV | 86 | 62 (56-71) | 70/16 | 65 (74.6) | 18/68 | 43 (50)/37 (43) | 7.7 |
| LEN | 146 | 62 (55-70) | 124/18 | 109 (74.6) | 14/132 | 76 (52.1)/91 (62.3) | 7.2 | ||
| Maesaka et al.2022 [15] | Japan, prospective# | ATE/BEV | 69 | 76 (49-93) | 53/16 | 36 (52.2) | 34/35 | 12 (17.4)/27 (39.1) | 9.1 (1.9-14.5) |
| LEN | 161 | 73 (50-91) | 126/35 | 95 (59) | 80/81 | 34 (21.1)/60 (37.3) | 9.4 (2.0-22.2) | ||
| Niizeki et al. 2022 [16] | Japan, retrospective# | ATE/BEV | 161 | 73 (38-93) | 123/38 | 85 | 83/74 | 35 (21.7)/47 (29.2) | 12.1 |
| LEN | 568 | 72 (31-93) | 467/101 | 318 | 265/275 | 106 (18.7)/204 (35.9) | 18 | ||
| Persano et al. 2022 [17] | Multicentric, retrospective | ATE/BEV | 823 | – | 657/166 | 442 (53.7) | 335/488 | NR/305 (37.1) | 10.4 |
| LEN | 1312 | – | 1032/280 | 1165 (88.8) | 554/758 | NR/477 (36.4) | 13.7 | ||
| Rimini et al. 2022 [18] | Multicentric, retrospective# | ATE/BEV | 190 | – | 149/41 | – | 85/105 | 144 (75.8)/NR | 8.9 |
| LEN | 569 | – | 457/112 | – | 235/334 | 107 (18.8)/NR | 13.7 | ||
| Su et al. 2022 [19] | Taiwan, retrospective* | ATE/BEV | 46 | 61 (38-84) | 38/8 | 41 | 14/32 | 24 (52.2)/15 (32.7) | 8.2 (5.1-12.0) |
| LEN | 46 | 69 (40-87) | 30/16 | 38 | 16/30 | 24 (52.2)/17 (37) | 10.5 (5.9-18.2) | ||
| Casadei-Gardini et al. 2023 [12] | Multicentric, retrospective* | ATE/BEV | 864 | 72 (65-79) | 690/174 | 473 (54.7) | – | 188 (20.3)/314 (36.3) | 11.1 (6.8-15.0) |
| LEN | 1341 | 72 (65-79) | 1054/287 | 779 (58.1) | – | 272 (20.3)/488 (36.4) | 13.8 (7.6-22.9) |
Table 2
Outcome of the individual studies included in the meta-analysis
| Author | Drug | No. of patients | Objective response rate (%) | Disease control rate (%) | Overall survival (months) | Progression-free survival (months) | Overall adverse events (%) | ≥ Grade 3 adverse events (%) |
|---|---|---|---|---|---|---|---|---|
| Hiraoka et al. 2023 [13] | ATE/BEV | 194 | 87/194 (44.8) | 164/194 (84.5) | NE (15.0-NR) | 8.0 (6.2-10.3) | – | – |
| LEN | 57 | 27/57 (47.3) | 49/57 (86.0) | NE (13.6-NR) | 6.8 (4.8-8.1) | – | – | |
| Kim et al. 2022 [14] | ATE/BEV | 86 | 30/86 (34.9) | 65/86 (75.6) | NE | 5.7 (2.1-9.3) | 67/86 (77.9) | 37/86 (43.0) |
| 25/78 (32.0) | NE | 5.7 (3.8-10.5) | ||||||
| LEN | 146 | 49/146 (33.5) | 112/146 (76.7) | 12.8 (6.7-18.9) | 6.0 (5.2-6.7) | 103/146 (70.5) | 32/146 (21.9) | |
| 29/78 (37.2) | 19.9 (9.8-19.9) | 7.3 (5.7-9.4) | ||||||
| Maesaka et al. 2022 [15] | ATE/BEV | 69 | 31/69 (44.9) | 53/69 (76.8) | NE | 9.1* | 68/69 (98.5) | 21/69 (30.4) |
| 29/66 (43.9) | 50/66 (75.7) | NE | 8.8* | |||||
| LEN | 161 | 75/161 (46.5) | 125/161 (77.6) | 15.5 | 5.3 | 161/161 (100) | 95/161 (59.0) | |
| 34/66 (51.5) | 54/66 (81.8) | 20.6 | 5.2 | |||||
| Niizeki et al. 2022 [16] | ATE/BEV | 161 | 71/161 (44.1) | 143/161 (88.8) | NE | 7.6* | – | – |
| 68/152 (44.7) | 137/152 (90.1) | NE | 8.3* | |||||
| LEN | 568 | 270/568 (47.5) | 458/568 (80.6) | 20.4 | 5.8 | – | – | |
| 70/152 (46.0) | 120/152 (78.9) | 20.2 | 6.0 | |||||
| Persano et al. 2022 [17] | ATE/BEV | 823 | 225/823 (27.3) | 653/823 (79.3) | 15.9 (14.7-23.9) | – | – | – |
| LEN | 1312 | 506/1312 (38.5) | 1054/1312 (80.3) | 16.3 (15.2-18.3) | – | – | – | |
| Rimini et al. 2022 [18] | ATE/BEV | 190 | 54/190 (28.4) | 174/190 (91.5) | 12.1 (11.1-16.8) | 5.5 (4.7-7.4) | 134/190 (70.5) | 1/190 (0.5) |
| 54/187 (28.9) | 153/187 (81.8) | |||||||
| LEN | 569 | 227/569 (39.8) | 501/569 (88.0) | 17.8 (15.8-43.8) | 7.7 (6.8-8.4)* | 441/569 (77.5) | 45/569 (7.9) | |
| 77/187 (41.2) | 152/187 (81.2) | |||||||
| Su et al. 2022 [19] | ATE/BEV | 46 | 19/46 (41.3) | 30/46 (65.2) | NE | 5.9 | 29/46 (63.0) | 5/46 (10.9) |
| LEN | 46 | 12/46 (26.1) | 29/46 (63.0) | 22.2 | 5.3 | 35/46 (76.1) | 11/46 (23.9) | |
| Casadei- Gardini et al. 2023 [12] | ATE/BEV | 864 | 221/864 (25.6) | – | 16.4 (8.8-NR) | 8.1 (3.5-15.5) | 603/864 (69.8) | 421/864 (48.7) |
| 16.4 (8.2-NR) | 8.2 (3.6-15.0)* | |||||||
| LEN | 1341 | 486/1341 (36.2) | – | 16.1 (8.6-44.0) | 6.3 (3.2-12.3) | 1140/1343 | 921/1343 (68.5) | |
| 15.8 (8.4-44.0) | 6.3 (3.1-12.3) | (84.9) |
Table 3
Study quality assessment for individual studies using the Newcastle-Ottawa Scale for cohort studies
| Study | Selection | Comparability | Outcome | Overall quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort Ascertainment of exposure | Assessment of outcome | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts based on the design or analysis controlled for confounders | Assessment of outcome | Whether follow-up was long enough for outcomes to occur | Adequacy of follow-up of cohorts | ||||
| Hiraoka et al.2023 [13] | * | * | * | * | ** | * | * | Good | |||
| Kim et al. 2022 [14] | * | * | * | * | ** | * | Poor | ||||
| Maesaka et al.2022 [15] | * | * | * | ** | * | * | * | Good | |||
| Niizeki et al.2022 [16] | * | * | * | * | ** | * | * | Good | |||
| Persano et al.2022 [17] | * | * | * | * | ** | * | * | * | Good | ||
| Rimini et al. 2022 [18] | * | * | * | * | ** | * | * | * | Good | ||
| Su et al. 2022 [19] | * | * | * | * | * | * | Poor | ||||
| Casadei-Gardini et al. 2023 [12] | * | * | * | * | ** | * | * | * | Good | ||
Objective response rate
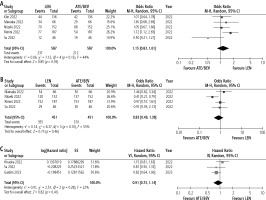
All the included studies (N = 6633) reported on the difference in ORR between the two therapies. In the unmatched cohorts, LEN was associated with a better ORR compared to ATE/BEV with OR 1.34 (95% CI: 1.10-1.63, I2 = 56%). However, in the analysis of matched cohorts from five studies (n = 1174) [13-15, 17, 18], the ORR was comparable between the two groups (aOR = 1.15, 95% CI: 0.83-1.61, I2 = 44%) (Fig. 2A).
Disease control rate
Seven studies (n = 4428) [12-18] and four studies (n = 902) [14, 15, 17, 18] reported the difference in DCR, respectively. There was no difference in DCR between LEN and ATE/BEV groups in both unmatched (OR = 0.91, 95% CI: 0.72-1.13, I2 = 21%) and matched cohorts (aOR = 0.83, 95% CI: 0.49-1.38, I2 = 53%) (Fig. 2B).
Overall survival
All eight studies reported the difference in OS between the two groups, while five reported OS after adjustment. There was no reported difference in the OS between the two groups in any of the included studies (Table 2). However, a meta-analysis of HR could not be performed due to the non-availability of data on HR.
Progression-free survival
Seven studies reported the difference in PFS between the two groups [12-15, 17-19]. Three studies reported a significantly longer PFS with ATE/LEN [14, 15, 19], while one reported a longer PFS with LEN [17] (Table 2). On analysis of aHR available from three studies, there was no difference in the PFS between LEN and ATE/BEV groups (HR = 0.91, 95% CI: 0.73-1.14, I2 = 22.0%) (Fig. 2C).
Adverse events
The incidence of any AE or ≥ grade 3 AE was reported by 5 studies (n = 3520) [13, 14, 17-19]. There was no difference between LEN and ATE/BEV in terms of overall AE, with OR = 1.55 (95% CI: 0.92-2.63, I2 = 78%). Concerning ≥ grade 3 AE, the two groups were comparable with OR = 2.09 (95% CI: 0.85-5.13, I2 = 90%).
Publication bias and sensitivity analysis
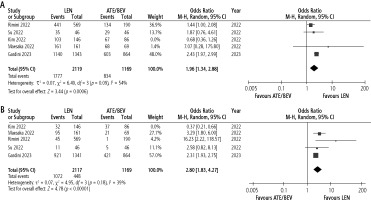
There was no evidence of publication bias for any of the evaluated outcomes except for ORR in unmatched cohorts. When the study by Kim et al. [14] was excluded, LEN was associated with a higher incidence of AEs (Fig. 3A) and ≥ grade 3 AEs (Fig. 3B) compared to ATE/BEV.
Discussion
The current BCLC guideline recommends ATE/BEV as the first-line therapy along with durvalumab and tremelimumab combination for managing aHCC, with LEN or SOR being reserved for cases not suitable for the above. This recommendation was made based on the results of a single randomized controlled trial (RCT) [8], and no head-to-head RCTs have compared ATE/BEV with LEN. Similarly, the recently updated guidelines from the European Society of Medical Oncology (ESMO) also recommend ATE/BEV as the first-line therapy, with alternative options being LEN and sorafenib. The National Comprehensive Cancer Network (NCCN) guidelines have also positioned ATE/BEV as the first-choice therapy in advanced unresectable HCC, with other first-line options being LEN, sorafenib, and in specific circumstances, nivolumab and FOLFOX. Based on available data, ATE/BEV and LEN appear to be the most promising agents as first-line therapy in aHCC, and choices may depend on real-world scenarios. Hence, the present meta-analysis was conducted to compare the outcome ATE/BEV and LEN in aHCC, which reported a comparable ORR (aOR = 1.15, 95% CI: 0.83-1.61) and DCR (OR = 0.83, 95% CI: 0.49-1.38) in matched cohorts. Similarly, there was no difference in the PFS between the groups (aHR = 1.06, 95% CI: 0.75-1.50).
In a previous network meta-analysis of 27 RCTs comparing first-line systemic therapies for aHCC [22], LEN was ranked as the best therapy among all treatments, followed by ATE/BEV concerning ORR, but without any significant difference (RR = 0.80, 95% CI: 0.44-1.45). Concerning PFS, there was no significant difference between ATE/BEV and LEN with HR 0.89 (95% CI: 0.64-1.25). However, ATE/BEV was associated with a better OS than LEN (HR = 0.63, 95% CI: 0.44-0.89). LEN was ranked higher for grade ≥ 3 AEs than ATE/BEV. Consistent with the previous indirect analysis results, the present direct comparison showed a comparable ORR and PFS between ATE/BEV and LEN and a higher incidence of overall AEs and grade ≥ 3 AEs with LEN.
Multiple factors can influence the efficacy of the drugs as both drugs have a different mechanism of action. LEN acts on various targets in HCC pathogenesis, such as vascular endothelial growth factor receptor, fibroblast growth factor receptor, plateletderived growth factor receptor, and cKIT [23]. ATE (anti-PDL1) and BEV (anti-VEGF) have fewer but important molecular targets compared to LEN [24]. In a previous meta-analysis, patients with HCC and viral etiology of cirrhosis showed a benefit from checkpoint inhibition (HR = 0.64, 95% CI: 0.48-0.94), whereas patients with HCC of a non-viral etiology did not (HR = 0.92, 95% CI: 0.77-1.11) [25]. The authors concluded that non-viral HCC, particularly non-alcoholic steatohepatitis (NASH)-related HCC, is less sensitive to immunotherapy because tissue damage resulting from aberrant T cell activation in NASH compromises immune surveillance. A proinflammatory microenvironment combined with fatty acid-mediated cytotoxic action also produces immune exhaustion in NASH-related HCC [25]. In the study by Casadei-Gardini et al. [12], though there was no survival advantage of ATE/BEV over LEN, a survival benefit of LEN over ATE/BEV was seen in patients with NASH-related HCC (HR = 1.88, 95% CI: 1.16-3.01), whereas ATE/BEV had a survival benefit in patients with viral etiology of cirrhosis (HR = 0.76, 95% CI: 0.61-0.96). A similar benefit with LEN in terms of PFS and OS among the nonalcoholic fatty liver disease (NAFLD)/NASH population was also noted by Rimini et al. [18]. In a study by Kim et al. [14], patients with baseline AFP > 200 ng/ml treated with ATE/BEV had a longer PFS compared to the LEN group (11.8 vs. 5.5 months, p = 0.047) with HR = 0.565 (95% CI: 0.322-0.922). Thus, the patient profile should be taken into consideration when choosing the optimal first-line therapy for aHCC.
A study by Maesaka et al. [15] revealed that hepatic reserve was preserved in the ATE/BEV group, while hepatic reserve deteriorated over time in the LEN group. This was reflected by a decrease in modified albumin-bilirubin grades (ALBI) over time in patients treated with LEN compared to those treated with ATE/BEV. This decline in hepatic function in the early stage of LEN initiation (within 2-4 weeks) was previously also reported by Hiraoka et al. [13]. The decline in hepatic reserve was seen to correlate with high baseline angiopoietin-2 (ANG2) and low VEGF serum levels at eight weeks after LEN initiation [12]. This points to the importance of starting LEN in patients with good hepatic function.
Apart from the efficacy of the drug, the cost of treatment is also a deciding factor for the choice of therapy and is relevant to third-world countries, where the cost of ATE/BEV is 15-20 times higher than LEN. In a cost-effectiveness analysis of five systemic treatments for unresectable HCC in China, donafenib was found to be the most economical option, followed by sorafenib > LEN > sintilimab plus bevacizumab > ATE/BEV [26]. Subsequently, Sun et al. conducted a network meta-analysis with a cost-effective analysis of the first-line treatments for aHCC in China and the United States [27]. Compared to donafenib, ATE/BEV and LEN added 0.46 and 0.77 quality-adjusted life-years. The authors concluded that LEN was the first-line treatment choice for Chinese aHCC patients and US payers’ perspectives. Thus, LEN is a cost-effective option compared to ATE/BEV for treating aHCC. However, we could not perform a direct, cost-effective analysis of the two therapies due to a lack of data from the included studies.
There was no difference between LEN and ATE/BEV in terms of overall AE and grade 3/4 AEs in the overall analysis. However, in leave-one-out analysis, LEN was associated with both higher incidence of overall AEs and grade 3/4 AEs, compared to ATE/BEV. Lenvatinib has been reported to be associated with a higher incidence of hypothyroidism, hand-foot skin reactions, and diarrhea compared to ATE/BEV, where hypertension, gastrointestinal bleeding, and perforation were common [12-14, 16, 19]. Maesaka et al. [15] reported that patients with any grade of proteinuria or hypertension on ATE/BEV had significantly longer median PFS than those without any grade of proteinuria or hypertension. In contrast, such a phenomenon was not seen in patients treated with LEN.
The present meta-analysis is the first to compare the outcome of LEN with ATE/BEV in the management of aHCC. Despite this, there are multiple limitations to the present analysis. The majority of the studies were retrospective and were prone to bias. Adjusted data were not available in all the studies. We could not perform a meta-analysis comparing OS between the two therapies due to a lack of data. We could not perform a cost-effective analysis comparing ATE/BEV and LEN due to unavailability of data in the included studies, and this remains a topic of future research.
To conclude, based on currently available data, primarily stemming from retrospective real-world studies, LEN appears non-inferior to ATE/BEV in ORR, DCR, OS, and PFS in adequately matched cohorts. However, LEN may be associated with a higher incidence of AEs. Treatment choice should be personalized based on the etiology of HCC and patient profile. There is an urgent need for well-designed prospective randomized trials for an appropriate comparison.