Introduction
Allergic diseases are currently an important health problem among children and adolescents. They are the most common chronic disorders affecting the youngest patients. The prevalence of allergic diseases in 6- to 7-year-old children in Poland was estimated to be 40% in multicentre randomized epidemiological research [1]. Atopic dermatitis (AD) is considered the most common chronic inflammatory disorder in children, with a prevalence up to 20% [2]. The beginning of symptoms usually occurs before the end of the second year of life [3]. AD is responsible for an important decrease in children’s quality of life and, at the same time, is a reason for high health care system costs [4–6]. Thus, investigation of the underlying mechanisms is crucial for further development of prophylaxis and therapeutic measures.
Despite many previous studies concerning the development of AD in children, the underlying mechanisms are not yet fully understood. An important knowledge gap in the field of allergic diseases is their connection with prematurity. Preterm infants are a special research group. The development of their immunological system may be disrupted by exposure to multiple environmental antigens, in contrast to that of children born full term. Many authors have drawn attention to the hypothesis of tolerance and sensitization to antigens during the development of a child’s immunological system [7, 8].
Aim
The connection between prematurity and AD has been intensively investigated. The last systematic review with a meta-analysis that summarized the advances in this specific field was published in 2018 and included articles published until February 2017 [9]. Since that time, at least five studies have been conducted [10–14], one of which had more than 1 million participants [10], and there have been advances in the comprehension of AD physiology, which is why an update on the current state of knowledge is highly necessary. The aim of this paper was to summarize what is already known and shed new light on what still needs to be clarified on the topic of the connection between gestational age (GA) and AD.
Material and methods
An electronic search of Medline was conducted using the keywords presented in Table 1. The abovementioned keywords were consecutively entered into the Medline search engine. The titles and abstracts of all the articles found were analysed to identify the relevant literature according to the inclusion and exclusion criteria. The search was time limited to the last 11 years (March 2011–March 2022). Only full-text English articles were considered. The selection criteria included research papers involving humans born prematurely and providing data about the development of AD at various stages of their lives. Subsequently, the full versions of the articles were screened to ensure the fulfilment of the selection criteria and to exclude duplicate papers. The detailed process of selection is shown in Figure 1.
Results
Thirteen articles met the inclusion criteria [8–20] (Table 2). Eight (n = 8) out of the thirteen studies included in the analysis confirmed a decreased risk of AD in premature children in comparison to term-born or post-term-born children. Five (n = 5) studies reported no association between these factors.
Table 2
Summary of the studies included in the review
| Study (year), location | Objective | Outcome definition | Sample size and design | Results |
|---|---|---|---|---|
| Studies that show the decreased risk of atopic dermatitis in premature patients (8 studies) (studies not included in the metanalysis by Zhu et al. are marked with*) | ||||
| Barbarot (2013), France [7] | To determine whether the risk of AD is influenced by preterm birth. | Presence of AD assessed through a standardized phone interview at 5 years or through a questionnaire mailed to parents after 2 years – in both cases a positive answer to the question, ‘Has a doctor ever told you that your child had atopic eczema?’ was defined as AD. | Prospective, observational n = 2675 2329 preterm babies, 346 term-born babies. | Lower gestational age (< 29 weeks) was significantly associated with a decreased risk of AD in the Epipage cohort (aOR = 0.57, 95% CI: 0.37–0.87; p = 0.009) and the LIFT cohort (aOR = 0.41, 95% CI: 0.18–0.90; p = 0.03); compared with higher GA (29–34 weeks) and full-term birth. |
| Trønnes (2013), Norway [15] | To explore the associations between preterm birth, asthma, and atopic dermatitis. | Cases of severe asthma and atopic dermatitis were identified among recipients of basic or attendance benefits in the National Insurance Scheme from 1967 through 2005 with International Classification of Diseases (ICD) codes. | Retrospective, observational n = 1,760,821 All live births in Norway from 1967 through 2001. | Compared with children born at term (37–41 weeks of gestation), preterm children were associated with decreased odds for severe atopic dermatitis (OR = 0.9, 95% CI: 0.8–1.0 for 32–36 weeks of gestation; OR = 0.7, 95% CI: 0.5–1.0 for 23–31 weeks). |
| Mitchell (2014)*, New Zealand [16] | To examine the association between birth weight and symptoms of asthma, eczema and rhinoconjunctivitis. | Current eczema was identified by a positive answer to a specific question in the questionnaire. | Multicentre, multicountry, cross-sectional study n = 421 543 participants Children (aged 6–7 years) were chosen from a random sample of schools in a defined geographical area. | Low birth weight was associated with a lower risk of ever having eczema. < 2.5 kg: OR = 0.88 (0.82, 0.96) 2.5–3 kg: OR = 0.94 (0.90, 0.99). |
| Egeberg (2016), Denmark [8] | To evaluate the potential neonatal risk factors, including gestational age, for the risk of developing AD in the first 5 years of life. | First occurrence of AD – based on the algorithm that analysed the retrospective data from the Danish National Patient Register. | Retrospective, observational N = 641834 All live births between 1997 and 2006. | Prematurity was inversely associated with AD risk (IRR = 0.74, 95% CI: 0.68–0.81), as well as low birth weight (IRR = 0.68, 95% CI: 0.61–0.75). The risk of AD was slightly increased in children with neonatal jaundice and those born in autumn and winter. No association between AD and UV was found. |
| Haataja (2016), Finland [17] | To evaluate the need for hospitalization due to asthma and atopic dermatitis and asthma medication reimbursement in moderately preterm and late preterm children compared to very preterm and term children up to 7 years of age. | Hospitalization due to asthma or atopic dermatitis was defined as a specific International Classification of Diseases (ICD) code recorded in the register. | Retrospective, observational n = 1018302 Children born in Finland between 1991 and 2008. | Hospitalization due to atopic dermatitis was more frequent among term children (5.2%) than among medium-preterm (4.2%) and late-preterm (4.7%) children. |
| Korhonen (2018)*, Finland [10] | To assess the incidence and risk factors for asthma and atopic dermatitis by seven years of age after early-term, full-term, late-term and especially post-term birth. | The authors analysed hospitalization due to atopic dermatitis. Hospitalization was defined as a specific ICD code recorded in the Hospital Discharge Register. | Retrospective, observational n = 1 039 263 All live-births in Finland from 1991–2008. | Post-term birth was associated with an increased risk (OR = 1.10, 1.06–1.15) for hospital visits due to atopic dermatitis. |
| Morata-Alba (2019)*, Spain [11] | To compare respiratory morbidity, atopy and asthma in preterm infants with a gestational age over 32 weeks and full-term infants. | The diagnosis of eczema was based on the guardians’ answers to the question from the International Study of Asthma and Allergies in Childhood (ISAAC) core questionnaire. | Prospective, observational n = 232 Children born in a tertiary hospital, preterm babies with a GA between 32 and 35 weeks and full-term infants. | 30.2% of the preterm infants and 44.8% of the full-term infants were diagnosed with atopic dermatitis, whereas at 7–8 years of age, 7.8% of the preterm and 29.3% of full-term babies had the same diagnosis, respectively (44.8% vs. 30.2%, p = 0.021; 29.3% vs. 7.8%, p < 0.001). |
| Pagano (2021)*, Italy [12] | To explore the neonatal risk factors and to describe the clinical manifestations of atopy in a cohort of preschool children born preterm. | Three researchers unaware of the study aims assigned a diagnosis of AD, wheeze and FA during the paediatric visit scheduled in the follow-up program. | Prospective, observational n = 165 Neonates with a gestational age < 32 weeks or a birth weight < 1500 g. | The gestational age was significantly higher in cases with AD than in controls (p < 0.05). |
| Studies that showed no association between the risk of atopic dermatitis and gestational age (3 studies) | ||||
| Kwinta (2013)*, Poland [18] | To determine if extremely low birth weight (ELBW) infants were at higher risk of the development of allergic and respiratory symptoms. | The primary outcomes – the presence of respiratory and allergic problems was defined as a positive response to the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire. The secondary outcome variables were positive results for a serum total immunoglobulin E level or skin prick test and the subject’s respiratory status determined by spirometry results and fractional exhaled nitric oxide (FeNO). | Retrospective, observational n = 121, ELBW infants discharged from the neonatal intensive care unit (NICU); the control group included age-matched children from one general practitioner’s office. | There was no difference between the groups in the occurrence of ever having eczema or having eczema symptoms in the last year. |
| Kahr (2014)*, Denmark [19] | To study whether early-life exposures, including gestational age, modify the genetic influence on atopic diseases in a twin population. | An atopic disease diagnosis was identified by answers to the specific question in the questionnaire. | Retrospective, observational n = 10 809 All live-born twins in Denmark registered in the Danish Twin Registry between 1994 and 2000. | None of the factors, including prematurity, were significantly associated with atopic dermatitis. |
| Nagasaki (2021)*, Japan [13] | To examine the similarities and differences in the age-specific association of asthma, allergic rhinitis/conjunctivitis, and atopic dermatitis with early-life infection-related factors and developmental adaptations. | The incidence of atopic dermatitis was based on a medical doctor’s diagnosis of the disease, which was reported by a family member. | Longitudinal, cohort study n = 47015 A birth cohort of The Longitudinal Surveys of Babies Born in the 21st Century by the Japanese Ministry of Health, Labour and Welfare. | There was no significant association between preterm birth and atopic dermatitis in any of the follow-up points. |
| Schoch (2021)*, USA [14] | To explore infant and environmental factors that may contribute to the association between prematurity and atopic dermatitis. | A child was classified as having atopic dermatitis if ICD codes for atopic dermatitis were recorded in the Electronic Health Records. | n = 4016, mother-infant dyads, retrospective study | Infants had a higher risk of developing AD if they had a longer gestation (p = 0.001), were delivered vaginally (p = 0.013), did not stay in the NICU (p < 0.001), or had a higher birth weight (p = 0.002). However, in modelling atopic dermatitis with the predictor variables, only NICU length of stay remained significantly associated with a lower risk of AD (p = 0.004). |
| Taylor-Robinson (2016)*, UK [20] | To identify early-life risk factors for eczema, and to explore how early-life risk factors explain any differences in eczema. | The diagnosis of eczema was based on the mother’s answer to the question from the ISAAC core questionnaire. | n = 14499 Children from the United Kingdom Millennium Cohort Study. | There was no significant effect on an eczema diagnosis associated with preterm birth. |
Nine (n = 9) publications analysed in this article were not included in the meta-analysis by Zhu et al. in 2018, either due to a publication year later than 2017 or because of the other inclusion criteria [10–19]. Four (n = 4) publications proved that prematurity was a protective factor against AD [10–12, 19] (including 2 large studies with over 400 000 participants [10, 19]), and five (n = 5) showed no association [13–17]. The details of the methodology are shown in Table 2, and the conclusions are discussed below.
In the study by Morata-Alba et al. comparing children born at term and between 32–35 weeks of gestation, the number of children diagnosed with atopic dermatitis was significantly higher in the patients born at term [11]. Similar outcomes concerning low birth weight, but not prematurity itself, were presented in the multicentre study performed in 26 countries with a large number of patients [19]. Low birth weight (< 2500 g) was associated with a decreased risk of eczema; however, the size of the decrease was not significant. Moreover, low birth weight was associated with a reduced risk of eczema in affluent countries but not in nonaffluent countries [9]. Further analysis of this phenomenon in subgroups of term-born children is shown in the publication by Korhonen et al., who investigated the frequency of hospitalization due to AD up to the age of 7 years in groups of children born early-term (37+0-38+6 weeks of gestation), full-term (38+0-39+6 weeks), late-term (40+0-41+6 weeks) and post-term (42+0 weeks and more) [10]. According to the results, hospital visits due to AD were most common in children born post-term in comparison to the whole investigated population. In the study by Pagano et al., children born before the 29th week of gestation and between 29 and 32 weeks of gestation were compared, showing a reduction in AD risk in the group of children born before 29 weeks of gestation (p < 0.05) [12].
There are also publications that do not support the hypothesis of an association between gestational age and AD. In the twin-based population study by Kahr et al., none of a variety of early-life exposure factors, including gestational age, were significantly associated with AD. The overall contribution of genetic factors to the susceptibility to AD was high (94%, 81–96%) [19]. Kwinta et al. compared ELBW patients with a control group of full-term-born children at a mean age of 6.7 years and found no significant difference between the groups in the occurrence of AD ever or in the last year [18]. However, these symptoms were less frequent in the ELBW group. In the United Kingdom Millennium Cohort Study, which mostly researched the socioeconomic factors influencing the development of AD by the age of five, preterm birth was not significantly associated with the diagnosis of eczema [17]. In a large population study by Schoch et al. that analysed the association of various perinatal factors with the development of AD, vaginal delivery mode, a lack of NICU stay, and a higher gestational age and birth weight were significantly associated with an increased incidence of AD, although in modelling the predictor variables of delivery mode, gestational age, birth weight and length of stay in the NICU, only the length of stay in the NICU was related to the development of AD after adjusting for other variables in the model [14]. Nagasaki et al., in a recent large birth cohort study, found no significant association between preterm birth and AD at any of the follow-up points, including at 5.5, 7, 8, 9, 10 and 11 years of age [13].
Discussion
AD is a disease with a multifactorial origin. Genetic burden is an important determinant [16], similar to environmental factors. Recently, the prevalence of most allergic diseases, including AD, has increased. This increase was the highest in younger age groups [21], which may suggest that exposures before and during pregnancy and in early life are important determinants of a subject’s susceptibility to allergies and are worth investigating.
The results of the review show that the majority of analysed studies report a clear association of prematurity with AD. The reason for the fact that, in some publications, no association between these factors was found may lie in their different methodologies (study design, definition of AD, ethnicity of the investigated population – Table 2). This outcome is consistent with findings from a meta-analysis published a few years ago by Zhu et al. [9]. The authors showed, based on 18 studies published up to February 2017, that very preterm infants, compared to full-term infants, had a lower risk of AD in both unadjusted and adjusted analyses. The limitations of this paper, in comparison to the systematic review by Zhu [9], are that it has a less structured method of research qualification for analysis and a descriptive summary of the results. Nevertheless, it includes the most recent pieces of research, most of them with a large number of participants, and connects the findings with the current advances in the pathophysiology of AD. The differences in the studies qualified for the review between this study and that of Zhu et al. [9] lie in the differences in the qualification criteria, including the time span of publication, involving more databases, and more precisely described statistical methods in the latter study.
Pathophysiology of AD – recent findings
According to the latest reviews on the pathophysiology of AD, it is a chronic disease characterized by skin barrier disruption, inflammation and itching [22].
The skin barrier is a formation made of terminally differentiated keratinocytes. Its outermost layer is called the stratum corneum (SC) and consists of corneocytes surrounded by the proteins loricrin and involucrin, which are embedded in intracellular lipids [23]. The homeostasis of the SC is assured by filaggrin, an important protein that, together with keratin, strengthens the SC, and later in the process, is decomposed into natural moisturizing factors that reduce transepidermal water loss (TEWL) [23, 24]. In AD, the expression of filaggrin in keratinocytes is diminished, similar to the level of ceramides in the epidermis. These protective lipids may also have an altered composition and structure compared to healthy skin [22, 25]. A newly discovered enzyme, sphingomyelin deacylase, may be responsible for the decreased level of ceramides in the epidermis of skin with AD [26]. Genetic factors play an important role in the pathogenesis of AD, with heritability up to 75%. Most importantly, filaggrin mutations were proven to increase the risk of AD by 2.5–3 times at the ages of 6 and 12 months [27]. Additionally, tight junctions (TJs), which are intercellular barriers in the epidermal granule layer which regulate the permeability of the skin, are key players in the skin barrier, with claudin-1 (CLDN1) being one of the most important adhesion proteins of TJs [22]. In patients with AD, CLDN1 single nucleotide polymorphisms or reduced expression have been proven [28]. Other genetic factors also play an important role in the pathogenesis of AD, including Ovo Like Transcriptional Repressor 1 (OVOL1), Small Proline Rich Protein 3, Mattrin (TMEM79), Serine Peptidase Inhibitor Kazal Type 5 (SPINK5) and Kallikrein-related peptidase 7 (KLK7) [29].
The abovementioned pathological changes result in gaps in the skin barrier, enabling the penetration of allergens, and are a starting point for the process of inflammation in the deeper layers of the skin.
Premature skin and risk of AD
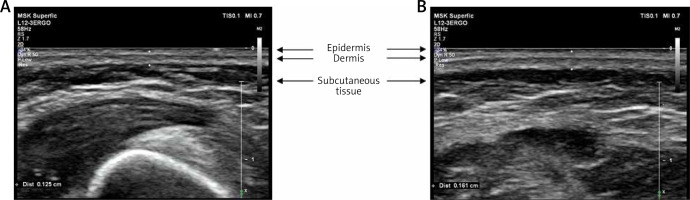
As the skin of a premature new-born differs substantially from that of a term-born new-born, it is possible that these differences are associated with a reduced risk of developing AD (Table 3). In a preterm neonate, stratum corneum maturation occurs at approximately 29–37 weeks of gestational age [30]. The formation of the skin barrier after birth may be quick because of the dry environment and NICU procedures, but TEWL is still much higher up to 34 and 35 weeks of gestational age [31]. Subsequent milestones in skin development are the appearance of rete ridges in the 30th week of gestation and the generalization of basketweave keratin at approximately the 28th week of gestation. All layers of the skin become thicker with gestational age, which has been shown by both autopsy and ultrasonographic (USG) methods [32, 33]. The comparison of preterm and term-born patients’ skin on ultrasound images is shown in Figure 2 (own, unpublished data).
Table 3
Differences in preterm and term-born patients’ skin, based on the studies of Saitoh et al. [33], Visscher et al. [31] and Reed et al. [32]
| Feature | Preterm | Full term |
|---|---|---|
| Skin thickness | Lower Mean skin thickness at thigh of 1.01 mm [33] | Higher Mean skin thickness at thigh of 1.15 mm [33] |
| Epidermal barrier | Less cornified layers | More cornified layers |
| Mechanical properties | Poor stratum corneum integrity, skin deficient in structural proteins, easily torn | Skin more damage-resistant |
| Stratum corneum maturation | Quick due to the dry environment and NICU practices | Already well formed |
| Vernix caseosa covering | Absent | Present |
| TEWL at birth | High | Low |
| Rete ridges | Absent before 30 weeks of gestation | Present |
| Transition from compact to basketweave keratin | Less prevalent | More prevalent |
Figure 2
Representative ultrasound images of the skin of preterm and term-born patients. A – Skin of a 2-week-old premature baby, born in 26th week of gestation. Thickness of epidermis and dermis – 0.125 cm. B – Skin of a 3-day-old term-born baby, born in 38th week of gestation. Thickness of epidermis and dermis – 0.161 cm
There were also differences in the skin surface cytokine levels between the groups, and the preterm infants were proven to have higher levels of IL-1β, IL-6, MCP1, and IL8 [34]. The skin of preterm patients also lacks vernix caseosa, which, in full-term children, waterproofs, moisturizes and lubricates their skin [31]. Moreover, the environment after preterm birth differs substantially from the stable exposure to amniotic fluid during pregnancy, which may influence factors such as skin pH or TEWL [35]. Due to this and based on studies with disinfectants [36], it has been suggested [37] that permeability of premature patients’ epidermis for various antigens may be increased in comparison to term-born patients and that skin barrier function is regarded as impaired in infants born before 29 weeks [7]. Moreover, such a phenomenon may cause constant, increased stimulation of the immune system by environmental saprophytes and other antigens via the skin, which is regarded to be necessary for inducing immune tolerance by activating regulatory T cells and dendritic cells [38].
It is, however, not completely clear why the disruption of the skin barrier in a preterm new-born would induce tolerance, while in older children, it could promote allergic sensitization with the subsequent development of AD. It has been suggested [14] that the timing of antigen exposure may be crucial as has already been demonstrated in animals. According to Scharschmidt et al. [39, 40], tolerance in mice develops in the second week of life, when Treg cells migrate to the skin in response to the microflora on the skin. Contact with skin commensals outside of this specific time period leads to inflammation instead of tolerance. It is however unknown whether such a critical window exists for humans.
Another difference between the skin of preterm and term children is the composition of cutaneous microbiota [41]. Staphylococcus aureus is described to be the more dominant species in preemies compared to other infants [42]. Interestingly, it was shown in a study by Kennedy et al. that early colonization with commensal staphylococci was associated with a lower risk of AD at 1 year of age [43]. Some authors suggest that altered microbiota of the skin may cause local immune reactions that prevent AD [37].
Inflammation and cytokine imbalance
Apart from skin barrier disruption, another element of AD pathophysiology is chronic inflammation with the overproduction of certain cytokine profiles [22]. According to the current state of knowledge, interleukin-25 (IL-25), interleukin-33 (IL-33) and thymic stromal lymphopoietin (TSLP) are present in skin with an impaired barrier and are responsible for enhancing type 2 inflammation and the overproduction of Th2-profile interleukin-4 (IL-4) and interleukin-13 (IL-13) [22, 44]. Additionally, CCL (CC chemokine ligand), CCL17, CCL18, CCL22 and CCL26, chemokines related to IL-4 and IL-13, are also overexpressed in the affected skin in patients with AD [45]. In the mechanism of a vicious cycle, filaggrin and loricrin expression is decreased by IL-13 and IL-4 [46]. Th2 cytokines were also shown to be able to change the metabolism of skin lipids [47].
In a foetus, the immune system is regulated by the mother’s immune responses [48] and bears a predominance to the T-helper 2 (Th2) cells. As this polarization is also observed in AD, as mentioned above, the question was raised whether changes in the T-helper 1 (Th1)/Th2 balance in premature children may be responsible for the decreased risk of AD in these patients [18]. It has been hypothesized that a shorter period of exposure to Th2 cytokines during pregnancy could result in a decreased risk of developing type 2 inflammation in the future [8, 9]. Furthermore, thymus weight may be smaller in post-term infants than in preterm infants, which can change the balance of Th2 and Th1 cell populations in the thymus in favour of Th2 cells, which are involved in the AD inflammation process [7, 49].
Another aspect is the immunological differences between preterm and term neonates concerning antibody development. Premature children produce fewer antibodies with lower antigen affinity, and their immunoglobulins maintain foetal characteristics, including short CDR-H3 regions [50]. Some authors suggest that this “foetal-like” low affinity antibody repertoire may last for years, changing the immune response of the children in the future [37]. Term new-borns also lack class-switched B cells at the time of labour, while premature patients express a diverse secondary antibody repertoire at the expected time of birth [37, 50].
Environmental factors
The other reasons for the lower risk of AD in preterm children may be environmental factors connected with the NICU stay. Proof for this thesis was provided by a recent study by Schoch et al. [14]. Exposure to humidified air in NICU incubators and the frequent use of emollients may be beneficial for skin barrier development. It was already shown that a humid environment may modify the genetic risk of AD [51]. Serious infections, which are common in the youngest patients, antibiotic exposure and nutrition differences may also be important factors [52]. It was also reported that time spent in the NICU may increase the risk of colonization with Staphylococcus in a dose-dependent manner as a part of the skin microbiome, which is probably associated with the pathogenesis of AD [14]. The reasons for these associations, however, have not yet been clarified.
In a study by Barbarot et al. and in a meta-analysis by Zhu et al., it was shown that the risk of AD was lower for very preterm children than for term-born children; however, there was no significant difference in the risk between children born moderately preterm and those born at term (29–36 weeks and 32–36 weeks of gestation, respectively) [7, 9]. The reason for such a discrepancy may exist because the period of extreme prematurity could be the “critical window” for the development of tolerance, as mentioned above; however, all these hypotheses require further investigation.
Post-term birth and the risk of AD
A few studies [8, 10] have reported that children born post-term have a greater risk of hospitalization due to AD than the rest of the investigated population. Post-term birth is acknowledged to be associated with serious birth complications, and it is also a risk factor for NICU admission and various inevitable neonatological procedures [53]. These children are exposed to a multitude of antigens immediately after birth, similar to preterm children. However, it is possible that their exposure happens outside of the abovementioned “critical window” for tolerance and, at the same time, causes sensitization to allergens instead of tolerance.
The implications of low birth weight on AD risk
In recent years, a few metanalyses have been published on similar topics, taking into consideration the connection of AD with low birth weight instead of prematurity. The results of Panduru et al. [54] showed that low birth weight may be considered a protective factor for AD. However, the authors did not adjust their statistics for gestational age, which in relation to abovementioned original studies may raise serious doubt if the effect is caused by abnormalities in weight itself – for example, by intrauterine growth retardation/prenatal hypertrophy (LGA) or by being born preterm/post-term. This doubt was nevertheless taken into account by Wooldridge et al. [55]. According to their systematic review that comprised only the studies corrected for gestational age, a 1 kg increase in birth weight was associated with a 17% greater risk of ever having AD in children and a 34% higher risk of ever having or currently having AD in patients up to 2 years of age after adjusting the results for gestational age. El-Heis et al. showed the link between foetal growth patterns and the pathogenesis of AD in their cohort study [56]. Higher femur length and abdominal circumference in pregnancy were proven to be protective factors, whereas disproportionate growth (a higher head to abdominal circumference ratio) and lower growth velocity increased the risk of AD. The authors suggest that these abnormalities may influence thymus development and the balance of T helper cells, but the mechanism still needs clarification. It is not completely clear which factor, gestational age, gestational growth or low birth weight, has a greater influence on AD risk; perhaps they all play a role in the pathogenesis of AD, as they share some risk factors and complications.
Conclusions
The main differences between the skin of preterm and term-born patients are the lower thickness of all the layers, an immature stratum corneum, a greater TEWL and a different cytokine profile and composition of keratin. According to eight out of the thirteen analysed studies, prematurity is associated with a lower risk of AD. The reason for the association of prematurity with a lower risk of AD may lie in the increased permeability of immature skin, the altered microbiota of the skin, and very early contact with antigens, especially those that are specific to a NICU stay. Reduced contact with the Th2-cytokine milieu during pregnancy in preterm babies may also act as a preventive factor for AD. The higher risk of AD in post-term children in comparison to that of term-born children still needs clarification. According to the current state of knowledge, both prematurity and low birth weight may be separate protective factors for the development of AD.