Introduction
Chronic spontaneous urticaria (CSU) is a heterogeneous disease characterized by short lived wheals with or without angioedema that last for more than 6 weeks. The diagnosis is based on clinical evaluation of typical skin changes. The wheals vary in intensity and duration, causing a significant influence on the quality of life and a substantial socio-economic burden. The aetiology and pathogenesis of CSU are still not completely understood. Autoimmunity, inflammation and coagulation processes are considered to play an important role in CSU pathogenesis [1].
Chronic spontaneous urticaria is associated with variable duration and response to therapy, so it is important to find biomarkers to assess disease activity that could help predict the disease duration and best treatment option. It is recommended to use urticaria activity score during 7 consecutive days (UAS7) as a disease assessment tool, a system that scores key urticaria signs and symptoms (wheals and pruritus), documented by the patient [2]. This is a highly subjective method, so disease activity is evaluated by the patient. There are no currently recommended biomarkers for disease activity, duration and treatment response in CSU patients.
Several studies have shown that patients with CSU have elevated levels of prothrombin fragment 1+2 (F1+2), D-dimer and FVIIa and that these markers correlate with disease activity [3–7]. It is found that serum concentrations of C-reactive protein (CRP), IL-6 and matrix-metalloproteinase 9 (MMP-9) are increased in urticaria patients and that they correlate with disease activity [8–10]. Kolkhir et al. [11] have reviewed available literature on blood biomarkers for chronic urticaria and strong evidence was found for 10 biomarkers (D-dimer, CRP, MMP-9, mean platelet volume (MPV), factor VIIa, F1+2, tumour necrosis factor, dehydroepiandrosterone sulphate and vitamin D) for distinguishing patients with CSU from healthy controls. Also, a significant association between CSU activity and D-dimer, F1+2, CRP, IL-6 and MPV was shown [11, 12].
Aim
Based on the results of previous studies that have found increased levels of certain biomarkers in CSU and correlation between biomarkers and disease activity, we aimed to evaluate some inflammatory (CRP), coagulation (prothrombin time (PT), activated partial thromboplastin time (aPTT), D-dimer) and immunological (C3, C4) parameters in CSU patients, compare them to healthy controls and to investigate the relationship between biomarkers and disease activity.
Material and methods
Patients
We enrolled 44 consecutive patients treated from June 2017 to July 2018 at the Clinic for Allergy and Immunology, University Clinical Centre of Serbia, who fulfilled EAACI/GA(2)LEN/EDF/WAO criteria for CSU diagnosis (spontaneous wheals with or without angioedema appearance daily or almost daily for ≥ 6 weeks, with no obvious external specific trigger). Exclusion criteria was the use of anticoagulation and antiplatelet therapy. The therapy for CSU was not stopped. The demographic data and personal history were recorded. The control group consisted of 33 healthy subjects. The study was approved by the local Ethical Committee and written consent was obtained from CSU patients and the controls (668/2).
Assessment of disease activity
Disease activity was assessed using UAS7. It quantifies the number of wheals (none: 0, mild: 1 (< 20 lesions in 24 h), moderate: 2 (20–50 lesions in 24 h), severe: 3 (> 50 lesions in 24 h)) and pruritus intensity (none: 0, mild: 1 (present but not annoying or troublesome), moderate: 2 (troublesome but not interfering with normal activity or sleeping) and intense: 3 (severe pruritus, which is sufficiently troublesome to interfere with normal daily activity or sleep)) on a daily basis for 7 consecutive days. Total score ranges from 0 to 42, allowing to classify disease activity as: well-controlled (0–6), mild (7–15), moderate (16–27), or severe (28–42).
Laboratory tests
Serum concentrations of CRP (turbidimetric, ARCHITECT), D-dimer (turbidimetric, BCS XP Siemens), C3 and C4 (turbidimetric, ARCHITECT), and PT and aPTT values (Sysmex CS-5100) were measured according to the manufacturer’s instructions. The reference ranges for CRP were 0–5 mg/l, for D-dimer 0–0.5mg/l FEU, for PT 10–13 s, for aPTT 22–32 s, for C3 0.83–1.93 g/l, for C4 0.12–0.36 g/l, respectively.
Statistical analysis
Statistical analyses were performed using SPSS for Windows, version 20. We used descriptive statistical methods (both numerical and graphical tools including mean, mode/median, percentage) and analytical methods (T-test, ANOVA, Spearman’s rank correlation coefficient). In all tests, p-values < 0.05 were considered significant.
Results
Forty-four patients, 38 (86.4%) females and 6 (13.6%) males, mean age of 50.4 (18–74) years and mean CSU duration of 3.1 years were enrolled in the study. Angioedema with urticaria was present in 24 (54.5%) patients. Fourteen (31.8%) patients had elevated CRP and 21 (47.7%) had elevated D-dimer. Increased C4 levels were found in 6 patients. Prolonged PT was present in 1 (2.3%) patient and prolonged aPTT also in one patient. In 1 patient, PT was decreased, and in 6.3% aPTT was decreased. Based on UAS7, more than half of CSU patients 23 (52.3%) had mild urticaria, 8 (18.2%) had well-controlled, 7 (15.9%) moderate and 6 (13.6%) of patients had severe urticaria (Table 1).
Table 1
Characteristics of CSU patients
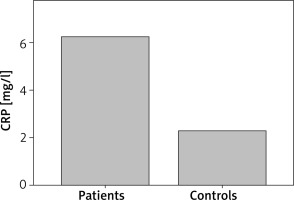
Patients with CSU had statistically significant elevated PT as compared with controls (p = 0.029). D-dimer was statistically significantly elevated in CSU patients as compared with controls (p = 0.007) (Figure 1). Also, CSU patients had statistically significant elevated CRP as compared with controls (p = 0.005) (Figure 2). There was no significant difference in aPTT between CSU patients and controls (p = 0.80).
Figure 1
D-dimer concentrations in CSU patients and controls. CSU patients have statistically significantly elevated D-dimer as compared with controls (p = 0.007)
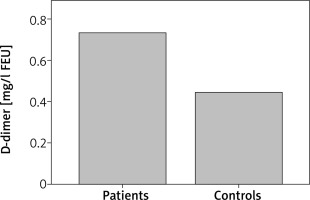
Figure 2
CRP concentrations in CSU patients and controls. CSU patients have statistically significantly elevated CRP as compared with controls (p = 0.005)
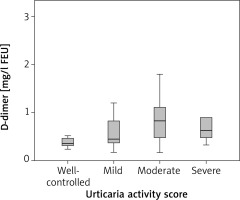
There was no correlation between D-dimer and disease activity (ρ = 0.293; p = 0.054). Mean D-dimer in patients with well-controlled CSU was 0.46 mg/l FEU, in mild urticaria 0.73 mg/l FEU, in moderate 0.85 mg/l FEU, and severe 0.98 mg/l FEU, but there was no statistically significant difference in D-dimer between these groups (p = 0.49) (Figure 3). No correlation was found between CRP, C3 and disease activity (ρ = 0.24; p = 0.105 and ρ = 0.037; p = 0.813, respectively). There were no statistical differences in CRP and in C3 levels between patients with well-controlled, mild, moderate and severe urticaria (p = 0.244 and p = 0.862, respectively). Prothrombin time and aPTT did not correlate with disease activity (ρ = –0.168; p = 0.247 and ρ = –0,178; p = 0.247, respectively). No difference was found in PT and aPTT between patients with well-controlled, mild, moderate and severe urticaria (p = 0.339, p = 0.339, respectively).
Figure 3
D-dimer levels in CSU patients with well-controlled, mild, moderate and severe urticaria. There was no difference in D-dimer between these groups (p = 0.49)
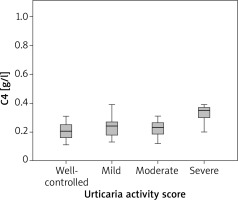
We found a correlation between C4 levels and disease activity (ρ = 0.32; p = 0.034). Analysis of variance has shown a statistically significant difference in C4 between patients with severe CSU and well -controlled, mild, moderate urticaria (p = 0.003). Further analysis has shown that patients with severe CSU had significantly higher C4 levels as compared with patients with well-controlled, mild and moderate urticaria (Figure 4).
Discussion
Chronic spontaneous urticaria is characterized by specific skin changes that usually do not present a diagnostic problem. However, the duration of CSU is very unpredictable, recommended therapy is often not effective, the aetiology remains unknown despite all investigations done and the pathophysiological mechanism is still not fully understood. Considering all these, there is a need for identification of biomarkers that would help to assess disease activity and to predict duration of the disease and response to therapy [13].
In recent years, many research studies evaluated activation of coagulation in CSU patients. Elevated D-dimer was present in 47.7% of our patients and was statistically significantly higher as compared with controls. Other studies have also shown increased D-dimer levels in CSU patients compared with controls in CSU patients compared with controls [14–16]. D-dimer is a marker of fibrin production and degradation of stable fibrin and reflects coagulation activation and fibrinolysis. Increased levels in our study are suggestive of the role of the coagulation process in the development of urticaria. Takahagi et al. [17] have observed that D-dimer may be the most sensitive biomarker to detect coagulation abnormalities in chronic urticaria, but fibrin degradation product (FDP) may be the most useful biomarker for disease activity. Based on the remark that levels of FDP and D-dimer are not different in patients with chronic urticaria, acute urticaria and angioedema, authors have suggested that coagulation activation may be a common phenomenon, not specific for chronic urticaria and might be a result of mast cell activation, rather than a primary event in wheal formation.
Our results revealed a significant difference in mean PT between patients and controls. Prothrombin time presents the time needed for blood to coagulate when tissue factor (TF) is added and it evaluates the extrinsic (and common) pathways of coagulation (FI, FII, FV, FVII, FX). Our findings indicate the role of the extrinsic coagulation pathway in CSU that is initiated by TF expression. Previous studies have found that TF from eosinophils in chronic urticaria activates extrinsic coagulation processes, with conversion of prothrombin into thrombin [18]. Thrombin acts on fibrinogen and produces fibrin that is degraded into FDP and D-dimer. Also, thrombin increases vascular permeability causing dermal oedema and stimulates mast cell degranulation [19] and may activate protease activated receptor 1 on mast cells [20]. However, we did not find the difference in aPTT between patients and controls (p = 0.80). Kim et al. [21] have shown prolonged aPTT in patients with chronic urticaria, but PT did not differ between patients and controls. Tests for PT and aPTT are important to evaluate initial pathways in coagulation, such as initiation of thrombin production and may indicate the importance of both intrinsic and extrinsic pathways in CSU.
We have found elevated CRP in 31.8% of CSU patients. Other studies have also shown increased CRP levels in 23–31% of CSU patients [17, 22]. Our patients had statistically significant elevated CRP as compared with controls, that is shown in other studies as well [14, 15]. C-reactive protein is a sensitive marker of inflammation, and as such is used as a biomarker of disease activity in many diseases, so it is not specific for CSU. However, current recommendations for CSU suggest that CRP should be done in all chronic urticaria patients [2]. Kolkhir et al. [22] have retrospectively analysed data from 1253 chronic urticaria patients and they have found that CRP was significantly higher in nonresponders to antihistamines as compared to responders, so CRP could act as a potential biomarker for therapy response. Also, it has been suggested that measurements of CRP may be useful in clinical practice to evaluate therapy response and disease exacerbation as it correlates with disease activity and severity [14]. However, clinical use of CRP should be considered as some patients with CSU have other diseases in which CRP could be also increased.
We did not show the correlation between coagulation (PT, aPTT, D-dimer) and inflammation (CRP) markers and disease activity and no difference was found in these parameters between CSU patients with well-controlled, mild, moderate and severe urticaria. There was an increase in mean D-dimer levels in patients with different levels of disease activity: D-dimer concentration in well-controlled urticaria was 0.46 mg/l FEU, in mild urticaria 0.73 mg/l FEU, in moderate 0.85 mg/l FEU, and in severe it was 0.98 mg/l FEU. Even though the trend toward higher D-dimer in patients with more active disease was observed, and results were at the cut-off point of statistical significance (p = 0.054), no correlation between D-dimer and urticaria activity was shown. The results of most other studies have shown that D-dimer correlates with urticaria disease activity and severity [4–7, 15, 17] as well as CRP [8, 9, 14, 16, 17]. Our results can be explained by the fact the most of our patients, 32 out of 44, were taking therapy for CSU, which could affect clinical presentation, CRP and D-dimer concentrations and UAS7 scoring. Also, more than half of CSU patients (52.3%) had mild urticaria, 18.2% had well-controlled, 15.9% had moderate and only 13.6% of patients had severe urticaria. In a study done in Poland, D-dimer, CRP, IL-6 concentrations were statistically higher in CSU patients as compared with controls, and a correlation between D-dimer and CRP, IL-6 and UAS was demonstrated [14]. All 58 patients in this study were without therapy for CSU and were classified into two groups based on modified UAS7 that was performed for 4 days: 8 had mild CSU (0–8 points) and 27 moderate-severe CSU (9–24 points). Many studies use UAS7 as a tool for disease activity evaluation, but there are no guidelines how to assess disease activity based on points gathered via this scoring system. It is not clear if disease activity reflects disease severity and vice versa as studies that evaluate biomarkers use both terms. This makes comparison of the results in different studies more difficult. In our study, we have classified CSU patients based on UAS7 into 4 groups: well-controlled, mild, moderate and severe CSU. Forty-four patients were enrolled and they were divided into 4 groups, so we can say that limitation of our study is the number of enrolled CSU patients.
We evaluated the role of the complement system in urticaria and have found a correlation between C4 levels and disease activity. Further analysis has shown a statistically significant difference in C4 between patients with severe and well-controlled, mild, moderate urticaria. Only patients with severe CSU had elevated mean C4 levels (0.40 g/l) and patients with well-controlled, mild and moderate had normal C4 levels (0.20 g/l, 0.23 g/l and 0.22 g/l, respectively). No correlation was found in C3 levels and disease activity. Kasperska-Zajac et al. [23] have evaluated C3, C4 and CRP in 70 urticaria patients, all therapy free, and they have classified disease activity into three groups (mild, moderate and severe). They found that serum C3 and C4 concentrations were significantly increased in patients as compared with the healthy subjects and those with moderate-severe symptoms showed higher C3 and C4 concentrations as compared with mild CU patients and healthy subjects. However, 90% of patients with severe urticaria had normal C4 levels and 95% had normal C3 levels, so despite severe disease activity, C3 and C4 concentrations were in normal range in most patients. They questioned the role of the complement system in urticaria, if increased serum C3 and C4 concentrations present the biomarker of acute phase response activation or they play an active role in urticarial processes. Another study has shown that 18 out of 35 patients with urticaria had low C4 levels and an association between reduction in C4 and positive autologous serum skin test were found, but with no clear relationship between them [24]. The role of C3 and C4 has not been explored extensively in CSU patients. Previous studies have shown that C5a, part of the complement system, is involved in mast cells activation in the course of CU [25]. The complement system is composed of many proinflammatory proteins, C4 being the major protein of the classical cascade. Elevated C4 may be the result of increased synthesis in the liver in response to cytokines IL-1β, IL-6 or tumour necrosis factor, which are increased in active urticaria [26]. Regarding these findings and our results, C4 may be an important player in active CSU, however its exact role still remains unknown.
In recent years, great research effort has been done to find reliable biomarkers in CSU. The problems with biomarkers in CSU are their specificity, usefulness in clinical practice and availability. It is not clear if inflammation, autoimmunity, coagulation processes in CSU may overlap or one play a dominant part in pathogenesis. It has been shown that markers of inflammation (IL-6, CRP) and coagulation/fibrinolysis (D-dimer) are related in CSU, and that there may be an interaction between these biological systems [14]. This suggests the existence of different phenotypes in CSU. The role of the biomarker is to identify these different groups of patients, to classify them according to the underlying pathophysiological mechanism and to find the best therapy option. More studies are needed in the future to resolve these issues.
Conclusions
CRP, D-dimer, and PT may be considered as biomarkers for distinguishing patients with CSU from controls. However, CRP, D-dimer, aPTT and C3 do not correlate with disease activity, but their involvement in CSU should be considered and further explored. Our results indicate the role of C4 as a marker of disease activity, but more studies are needed to evaluate the role of the complement system in urticaria. C-reactive protein and C4 are inflammation markers and they are increased in CSU patients, so inflammation may have an important part in CSU pathogenesis.