Introduction
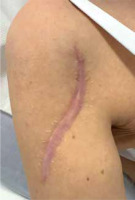
Keloids are fibrous scars that extend beyond the boundaries of a wound (Figure 1) [1]. According to the literature, they account for 11% of all scarring, with a significant prevalence among young individuals [2, 3]. These lesions are caused by various aetiologies, such as trauma and surgical procedures, but their development is also influenced by genetic factors [2, 4]. It is believed that their pathogenesis is associated with excessive fibroblast proliferation and migration, increased collagen synthesis and accumulation, as well as accelerated angiogenesis, which consequently leads to abnormal wound healing [5]. Their presence significantly exacerbates the medical condition of patients [4]. In addition to impacting physical appearance, keloids frequently cause pruritus, pain, and contribute to the deterioration of mental state [6, 7]. There are numerous therapeutic approaches to prevent and reduce keloids, with varying levels of efficacy [5, 8]. Botulinum toxin, particularly its most extensively utilized serotype A (BTX-A), is used for many purposes in medical and aesthetic practices. As evidence suggests, scar management is among the emerging applications of BTX-A [8]. Several reports in the literature pertain to the mechanism by which BTX-A may reduce the growth of keloids. These include inhibition of excessive proliferation of keloid fibroblasts and their differentiation into myofibroblasts, induction of fibroblast apoptosis, and prevention of muscle and skin contraction near keloid tissue [2, 4, 8, 9]. All of these processes lead to a reduction in symptoms and improvement in scar appearance [8].
The aim of the study was to explore the current evidence behind the use of botulinum toxin in the prevention and treatment of keloids.
Methods
A detailed literature review using PubMed database was conducted to evaluate toxin botulinum in the prevention and treatment of keloids. Studies published until 25 July 2024 were considered. A search was managed using the key terms ‘keloid scar’, ‘keloids’ and ‘botulinum toxin’. The information received from the above search was used in the compilation of the present article. Preliminary search yielded a total of 81 results (from 2019–2024), out of which 10 were selected after screening the titles and abstracts and reviewed. Animal or in vitro experiments were considered only for the purpose of investigating potential mechanisms explaining the formation of keloid scars and the action of BTX-A in their prevention and treatment.
The process of keloid scar formation
In the microscopic evaluation, keloids are characterized by densely packed but disorganized, type I and III collagen fibres [7]. In addition to those previously mentioned, the dysregulation of cytokine secretion has also been implicated as a contributor factor to keloid formation. Studies suggest that elevated levels of the proinflammatory interleukin (IL)-6 and IL-8, coupled with low levels of anti-inflammatory IL-10, may increase the risk [10]. Elevated levels of transforming growth factor-b (TGF-b) stimulate excessive activation of keloid fibroblasts and intensify extracellular matrix (ECM) collagen synthesis, which results in the keloid initiation process [6, 7]. It has been suggested that the elevated production of collagen by fibroblasts is attributed to the disparate production of TGF-b isoforms. The overexpression of TGF-b1 and TGF-b2, coupled with a decrease in the production of TGF-b3, leads to a rise in fibroblast activity and the production of collagen in the ECM. Moreover, TGF-b1 enhances the production of tissue inhibitors of metalloproteinases (TIMPs) and decreases the production of matrix metalloproteinases (MMPs), reducing the process of collagen degradation. The production of collagen is also stimulated by vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF). These cytokines have the potential to intensify fibroblast activation induced by mechanical stress in certain regions of the body (sternum, shoulders, and suprapubic regions) [7].
Furthermore, certain subtypes of human leukocyte antigens have been linked to a predisposition to keloids, indicating that their emergence is also the outcome of impaired immunological processes. Damage to the dermis triggers an immune response to sebum. This leads to the release of cytokines, which cause mast cell infiltration, and ultimately increased fibroblast activity. This could possibly account for the elevated probability of keloid formation in regions with a higher concentration of pilosebaceous units [10].
As the process of damage repair and proliferation progresses, fibroblasts gradually expand and transform into myofibroblasts. According to the study by Dai and Lei, keloid tissue is characterized by high expression of myofibroblast markers such as a-smooth muscle actin (a-SMA), collagen I, and collagen III. a-SMA accelerates wound healing, whereas collagen I and III enhance the ECM remodelling. Additionally, this study suggests that differentiation of human keloid myofibroblasts into adipocytes, by reducing ECM deposition, inhibits the keloid formation process [4]. Unlike hypertrophic scars, keloids are characterized by excessive tissue proliferation beyond the original wound margins, and their spontaneous regression is not observed over [8]. In addition, hypertrophic scars predominantly comprise of type III collagen [7].
Current treatment options for keloids
Despite numerous treatment options, there are no established guidelines for the management of keloids [3, 10]. Currently, intradermal injections of triamcinolone acetonide (TAC) are regarded as the gold standard for treatment, both as monotherapy and in combination. Studies have shown that with this treatment method, the regression rate is between 50% and 100%, and the recurrence rate is up to 50% after 5 years [5]. Corticosteroids may be also used in topical form. They reduce inflammation, limit oxygen delivery to the wound by constricting blood vessels, and have an antimitotic effect on keratinocytes and fibroblasts [10]. Their action is also associated with a reduction in the expression of a2-macroglobulin and a1-antitrypsin, i.e. collagenase inhibitors, the concentration of which is particularly high in scar tissue. Corticosteroids have limitations due to the risk of numerous complications resulting from prolonged use, such as local skin atrophy and telangiectasia [6]. Apart from corticosteroids, there are other topical therapies including retinoids, imiquimod, mitomycin C, and vitamin E [3, 5, 10].
Cryotherapy transforms keloid fibroblasts into fibroblasts with a normal phenotype by enhancing the ratio of type III to type I collagen. Radiotherapy (external beam radiotherapy and brachytherapy) can be administered at low or high doses. The use of this method after surgical excision of the keloid has been proven to be highly effective in reducing lesion recurrence. However, it is associated with adverse effects such as dyschromia and telangiectasia. Ablative lasers (Erbium laser (Er:YAG) and CO2 laser) and non-ablative lasers (ND:YAG, diode lasers, and pulsed dye lasers (PDL)) cause local damage to blood vessels and direct inhibition of fibroblasts. Photodynamic therapy, usually administered after the photosensitizing agent (5-aminolevulinic acid), is a potential adjunctive treatment option [10]. Surgical keloid excision is a possible treatment option for mature lesions after first-line therapy. This method alone is associated with a 45% to 100% recurrence rate, and is therefore used in combination with other therapies, including subsequent radiotherapy [3, 10]. Intradermal nonsteroidal injections encompass verapamil, 5-fluorouracil (5-FU), bleomycin, hyaluronidase, platelet-rich plasma (PRP), avotermin, IL-10, mannose-6-phosphate, insulin, and BTX-A, which will be extensively discussed in the subsequent paragraphs [5, 10].
Mechanism of action of botulinum toxin in the prevention and treatment of keloid scars
BTX-A paralyzes muscle tissue around the wound, reducing tension and contracture of elastic fibres during healing, which results in reduced inflammation and metabolic activity of cells, factors associated with scar hypertrophy [6, 8].
Studies have provided evidence for the regulatory role of BTX-A in fibroblast activity. It affects the expression of genes involved in keloidogenesis, increasing the expression of S100A coupled with the decrease in the expression of TGF-b1, connective tissue growth factor (CTGF), VEGF, MMP-1 and PDGF. These changes result in the inhibition of fibroblast proliferation. BTX-A regulates the fibroblast cell cycle and prevents fibroblast differentiation into responsive fibroblasts and myofibroblasts [6, 8]. According to the study by Dai and Lei, BTX-A promotes the transdifferentiation of primary keloid myofibroblasts into adipocyte-like cells via the activation of the bone morphogenetic protein 4 (BMP4)/Smad signalling pathway. It has been observed that BTX-A treatment increased the expression of peroxisome proliferator-activated receptor g (PPARg) and CCAAT/enhancer-binding protein a (C/EBPa) in keloid myofibroblasts, resulting in the accumulation of lipid droplets in these cells [4].
Growth arrest and DNA damage-inducing protein 153 (GADD153) is a major component of the endoplasmic reticulum stress-dependent apoptosis pathway. Its expression in cells is usually low. Factors such as nutrient deprivation, calcium ionophores, lipopolysaccharides, and genotoxic factors cause increased expression of GADD153. The regulation of GADD153 gene expression is mediated by c-Jun N-terminal kinase (JNK) and activator protein 1 (AP-1). Nien et al. showed that BTX-A induces GADD153 expression via the JNK and AP-1 pathway in cultured human keloid fibroblasts, resulting in their apoptosis [2].
Studies suggest that miRNAs are also involved in keloid progression. miR-1587 and miR-2392 inhibit keloid tissue formation, mainly by regulating fibroblast proliferation, inducing their apoptosis, autophagy, and epithelial-to-mesenchymal transition (EMT). In contrast, zinc finger E-box binding homeobox 2 (ZEB2), a target of miR-1587 and miR-2392, stimulates development of keloids. A study by Hou et al. showed that keloid tissue was characterized by significantly decreased expression of miR-1587 and miR-2392 and increased expression of ZEB2 compared with normal skin tissue. They also found that BTX-A caused increased expression of miR-1587 and miR-2392 and decreased expression of ZEB2 in keloid fibroblasts, indicating a possible inhibitory effect on the proliferation of these cells [11]. In addition, BTX-A markedly inhibited the expression of a-SMA, collagen I, and collagen III [4]. The results of the study by Fanous et al. confirmed that BTX-A was an effective treatment for keloid scars in an animal model [12].
The use of botulinum toxin in the prevention and treatment of keloids: new literature reports
There were found one prospective study [1], one meta-analysis [6], one interventional clinical trial [5], five randomised controlled trials [9, 13–16], and one retrospective study [17]. A summary of included manuscripts is presented in Table 1 [1, 5, 6, 9, 13–17].
Table 1
Summary of reports on the use of BTX-A in the prevention and treatment of keloid scars
| Study | Study group characteristics | Clinical response |
|---|---|---|
| Khatery et al. [1] | Patient group (N = 20) Age: 19.9 ±5.32 years Both sexes 12 patients with keloids 8 patients with hypertrophic scars | Statistically significant difference between the baseline value and the result after BTX-A injection sessions for VSS, OSAS and PSAS Statistically significant difference between the histopathological results before BTX-A injections and 1 month after the last session No complications |
| Liu and Zhang [6] | Meta-analysis | Combination therapy with corticosteroids and BTX-A is more effective and safer compared to corticosteroid injections alone |
| Gamil et al. [5] | Patient group (N = 50) Both sexes 26 patients received IL BTX-A injection in the right-sided lesions and IL TAC injections in the left-sided lesions Age: 28.4 ±6.5 years 24 patients received a combination of both BTX-A and TAC injections Age: 27.8 ±6 years | Combination therapy with TAC and BTX-A is more effective and safer compared to TAC or BTX-A injections alone |
| Rasaii et al. [13] | Patient group (N = 23) Age: 23.3 ±1.2 years Both sexes | Combination therapy with TAC and BTX-A significantly reduced pain and pruritus compared to TAC injections alone |
| Neinaa et al. [14] | Patient group (N = 60) Both sexes 20 patients received IL injections of BTX-A Age: 25.4 ±4.1 years 20 patients received IL injections of autologous PRP Age: 23.5 ±3.3 years 20 patients received IL injections of TAC Age: 26.1 ±4.2 years | Compared to TAC, BTX-A and PRP injections resulted in greater improvement in VSS score and better immunohistochemical results |
| Ismail et al. [15] | Patient group (N = 50) Age: 30.72 ±11.59 years Both sexes 22 patients were treated with IL BTX-A alone 22 patients were treated with IL 5-FU alone 6 patients were treated with both IL BTX-A for some lesions and IL 5-FU for the others | The use of IL BTX-A led to improved appearance of lesions, reduced symptoms, fewer side effects, no hyperpigmentation, and a lower recurrence rate compared to IL 5-FU |
| Sabry et al. [16] | Patient group (N = 20) Age: 12.5 ±3.3 years Both sexes 10 patients with keloids 10 patients with hypertrophic scars | In the case of keloids, the use of IL BTX-A led to better improvement of lesion vascularization and elasticity and lower POSAS scores compared to the combination of CO2 laser and topical BTX-A |
| Zhang et al. [17] | Patient group (N = 58) Both sexes Intervention group (N = 32) patients who received steroid injections and additional injection of BTX-A/HA on the same day Age: 24.1 ±3.2 years Control group (N = 26) patients who received only steroid injections Age: 25.5 ±4 years | Combination therapy with BTX-A, HA and corticosteroid injections led to reduced sebum production and TEWL, lower recurrence rates, better VSS and VAS, and increased satisfaction in patients with multiple sternal keloids |
| Tawfik and Ali [9] | Patient group (N = 15) Age: 7.2 ±4.2 years Both sexes | The use of BTX-A led to improvement in the vascularity, elasticity and height of lesions, reduction in itching and pain, and greater joint mobility limited by hypertrophic lesions Gradual improvement after each injection session was observed No complications |
[i] BTX-A – botulinum toxin type-A, VSS – Vancouver Scar Scale, OSAS – Observer Scar Assessment Scale, PSAS – Patient Scar Assessment Scale, IL – intralesional, TAC – triamcinolone acetonide, PRP – platelet-rich plasma, 5-FU – 5-fluorouracil, POSAS – Patient Observer Scar Assessment Scale, HA – hyaluronic acid, TEWL – transepidermal water loss, VAS – Visual Analog Scale.
All analysed studies confirmed the effectiveness of intralesional (IL) injections of BTX-A in keloid therapy. BTX-A prevented muscle contractions near the keloid, reducing the tensile force on the tissue, regulated fibroblast proliferation and TGF-b production. This led to a reduction in the size of the lesions, an improvement in their appearance, but also a reduction in the symptoms reported by patients. In these studies, different scales were used to evaluate clinical improvement: the Vancouver Scar Scale (VSS), which assesses scar vascularity, height/thickness, flexibility, and pigmentation [9], the Patient Observer Scar Assessment Scale (POSAS), which includes the Observer Scar Assessment Scale (OSAS) and the Patient Scar Assessment Scale (PSAS) [1], and the Visual Analog Scale (VAS). Additionally, one study estimated transepidermal water loss (TEWL) [17].
In their prospective therapeutic and pathological study, Khatery et al. examined 20 patients, 12 with keloids and 8 with hypertrophic scars, and found a statistically significant difference between the baseline value and the outcome after each of the three BTX-A injection (2.5 U/cm3 of each lesion) sessions and almost 4 months after the last session for VSS, OSAS and PSAS (p ≤ 0.001). A statistically significant difference was also observed between the histopathological results before BTX-A treatment and 1 month after the last session (reduction in the percentage of collagen area, increase in the percentage of elastic tissue area). No complications were noted [1]. A 2021 meta-analysis by Liu and Zhang found that compared with corticosteroid monotherapy, corticosteroid in combination with BTX-A is more effective in treating keloids and hypertrophic scars. BTX-A, in comparison to corticosteroids, is characterized by fewer side effects and greater safety of use. Combined therapy with BTX-A and corticosteroids had a beneficial effect on the thickness of lesions, contributed to reduced itching, better patient satisfaction and results in VAS and VSS scales [6]. Similarly, another study of 50 patients with keloids showed that the combination of TAC and BTX-A injections (2.5 U/cm3 of the lesion with a maximum dose of 100 U per session) produced better clinical outcomes (reduction in keloid thickness and area), with fewer adverse events compared with TAC or BTX-A monotherapy [5]. Rasaii et al. reported that combined therapy with BTX-A (maximum dose of 20 U per session) and IL TAC, compared to TAC monotherapy, significantly reduced physical symptoms (pain and pruritus) in patients [13]. Neinaa et al. compared the effects of BTX-A, PRP, and TAC injections in the treatment of keloids. They reported that the use of BTX-A (5 U/injection point with a maximum dose of 60 U per session) or PRP resulted in greater improvement of VSS and better immunohistochemical results (reduction in CTFG expression) compared with TAC injections [14]. Ismail et al. examined 50 patients with keloids. Twenty-two patients were treated with IL BTX-A alone (2.5 U/cm3 of the lesion with a maximum dose of 100 U per session), 22 patients with IL 5-FU alone, and 6 patients were treated with both IL BTX-A for some lesions and IL 5-FU for the others. A better clinical effect (flattening of the lesions), reduction of symptoms (less pain and itching), fewer side effects, no hyperpigmentation and a lower recurrence rate were observed in scars treated with IL BTX-A. The greater efficacy of IL BTX-A was noticeable especially in the case of large-sized keloids [15]. Another study by Sabry et al. evaluated the efficacy and safety of IL (2.5 U/cm3 of the lesion with a maximum dose of 100 U per session) and topical BTX-A combined with fractional CO2 laser in the treatment of hypertrophic and keloid scars in 20 patients. BTX-A was injected monthly (4 times in total) into one half of the scar, CO2 laser therapy was applied to the other half once a month (4 sessions in total), and then topical BTX-A. In keloids, IL BTX-A treatment led to better improvement of lesion vascularization and elasticity and lower POSAS scores compared to a combination of CO2 laser and topical BTX-A [16]. Zhang et al. used a combination of BTX-A and hyaluronic acid (HA) (2.1 ml solution containing 10 U BTX-A and 0.6 ml HA delivered per 100 cm2 of the injection area) with corticosteroid injections to treat patients with multiple sternal keloids. This therapy led to reduced sebum production and TEWL, lower recurrence rates, better VSS and VAS, and increased patient satisfaction compared to the control group [17]. In microtoxin therapy, BTX-A is diluted and injected intradermally into a specific skin area. Fabi et al. described the use of this form of therapy for various aesthetic problems, including the prevention of postoperative or posttraumatic hypertrophic scars and the treatment of existing keloids. The recommended dose of microbotox for these indications was 28 U/ml [18]. Hypertrophic and keloid scars are also a problem among children. It is estimated that these lesions may occur during burn wound healing in more than 20% of children under 5 years of age who have sustained a burn. In their randomised controlled trial, Tawfik and Ali showed that BTX-A is effective and safe in the treatment of hypertrophic and keloids in paediatric population. 15 children were examined. Each lesion was divided into two parts, one of which was injected with BTX-A (5 U/cm2 of the lesion), and the other served as a control. The use of IL BTX-A resulted in a better VSS, significant improvement in vascularity (erythema reduction) and elasticity, and a reduction in the height of the lesion, relief of itching and pain, and greater joint mobility previously limited due to hypertrophic lesions. The described improvement was noted after the first application of BTX-A and its degree increased after each session. No complications were noted [9].
Conclusions
BTX-A presents a promising approach for the treatment of keloids. Its use leads to significant histologic improvement of scar tissue. A reduction in erythema and scar thickness is observed, as well as a decrease in symptoms, such as itching or pain. It is imperative to use this method in combination with HA or corticosteroids, as it facilitates enhanced treatment outcomes with fewer adverse effects. Treatment of keloids with BTX-A has also shown safety in children. There is a need for further studies to establish the value of this therapeutic method in keloid treatment protocols, which may lead to improved clinical effects for patients.