Introduction
Liver cancer is the sixth most commonly diagnosed cancer and the third leading cause of cancer-related deaths worldwide with most cases detected at late stages [1, 2]. Hepatocellular carcinoma (HCC) represents about 75-85% of primary liver cancers, and mostly develops in fibrotic chronic liver diseases (CLD), usually in cirrhosis [3].
Serum α-fetoprotein (AFP) is a gold standard biomarker mostly used for screening of suspicious HCC lesions in patients with liver cirrhosis, and has been proven to have capability of prefiguring the prognosis [4]. However, studies showed that AFP has a low diagnostic sensitivity, particularly in diagnosis of early-stage HCC, and its level is raised in other cases of CLD [4, 5]. Despite the recent progress in HCC diagnosis and intervention over the last decade, the overall prognosis still remains unsatisfactory, and there is an urgent need to search for novel biomarkers with high efficacy for early detection of HCC and prediction of tumor progression [6]. Elucidating the molecular mechanisms of tumor growth would allow the development of effective diagnostics and therapeutics, and ultimately help patients with HCC to achieve a favorable prognosis [7]. Research studies demonstrated that non-coding RNA plays a role in regulating multiple biological processes in various cancers including HCC [8, 9].
Only 1.5% of the human genome is protein coding while the remaining ~98% is transcribed into noncoding RNA (ncRNA) that does not produce proteins [10]. Long ncRNA (lncRNA), which consists of > 200 nucleotides, promotes the proliferation, migration, invasion, autophagy and apoptosis of tumor cells by regulating downstream target gene expression and cancer-related signaling pathways [11, 12]. Some lncRNAs may act as “oncogenes” or “tumor suppressors” for cancers and are useful in tumor diagnosis and prognosis [13]. In patients with HCC, lncRNA dysregulation was highly associated with disease recurrence, metastasis and poor clinical outcomes [14, 15].
Homeobox (HOX) transcript antisense intergenic RNA (HOTAIR) is a 2.2-kb gene residing in the mammalian HOXC locus on chromosome 12q13.13. It has been considered to play critical roles in the biological properties of various tumors [16]. It was discovered as a gene repressor of HOXD genes, and demonstrated the important relationship between epigenetic regulation by lncRNA and cancer [17]. Specifically, HOTAIR recruits the polycomb repressive complex 2 (PRC2) occupancy and enhances the trimethylated histone 3 lysine 27 (H3K27me3) repressive marks [18]. It plays an important role in the development and progression of complicated diseases, including esophageal squamous cell carcinoma, colon cancer and breast cancer [19]. Hypoxia regulates HOTAIR to modulate cell proliferation, programmed death, invasion and migration [20].
Therefore, the present study was designed to investigate the clinical usefulness of serum HOTAIR as a potential biomarker for predicting HCC and prefiguring the tumor stage.
Material and methods
Study population
This is a cross-sectional study conducted at the Alexandria University Hospital and included 80 patients with definite newly diagnosed HCC on top of hepatitis C virus (HCV)-related liver cirrhosis, divided into 40 HCC patients with tumor stages C-D according to the Barcelona Clinic Liver Cancer (BCLC) classification (group IA) and 40 HCC patients with tumor stages 0/A-B according to the BCLC classification (group IB), and 40 cirrhotic patients without HCC (group II). Also, 20 healthy subjects with no evidence of liver disease were included as a control (group III). Exclusion criteria were non-HCC hepatic malignancy, extra-hepatic malignancy, other causes of liver disease, and previous surgical, locoregional or systemic therapies of HCC.
The study was conducted in accordance with the provisions of the World Medical Association Declaration of Helsinki. The research protocol was approved by the Ethics Committee of the Alexandria Faculty of Medicine (IRB. No. 00007555). Informed consent was obtained from all subjects included in the study.
Preliminary evaluation
The study participants were evaluated clinically as regards manifestations of liver cirrhosis and malignancy, with complete clinical examination and routine laboratory investigations including complete blood picture and liver test assay. Serum AFP levels were measured using enzyme-linked immunosorbent assay (ELISA) (Cortez Diagnostics Inc., Cat. No. 5101Z) [4]. Liver disease severity was determined on the basis of the Child-Turcotte-Pugh (CTP) classification. The diagnosis of HCC was confirmed by triphasic computed tomography (CT) and/or dynamic magnetic resonance imaging (MRI) of the liver showing contrast hyper-enhancement in the arterial phase “wash-in” and hypo-enhancement in the portal or delayed phase “wash-out”, with specific focus on the tumor characteristics (location, maximum diameter, number of nodules, tumor-in-vein thrombus and metastasis) [21]. The stage of HCC was determined according to the BCLC classification [22].
Measurement of serum HOTAIR level
For laboratory evaluation of circulating HOTAIR expression, 10 ml of venous blood was withdrawn from every patient and control subject. Each blood sample was allowed to clot, then centrifuged at 1200 × g for 10 minutes to separate the serum sample, which was kept frozen at –80°C until use. Total RNA isolation from serum samples was performed using the Qiagen miRNeasy Mini-Kit (Applied Biosystems Inc., Cat. No. 217004). The concentration and purity of RNA were measured using nanodrop then complementary DNA (cDNA) was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., Cat. No. Archive) [11]. The thermal cycle was programmed at 10 minutes hold at temperature 25°C, 120 minutes hold at temperature 37°C, 5 minutes hold at temperature 85°C, then lowering the temperature to 4°C and stopping the run. Each reaction comprised approximately 10 µg of RNA extract, 2 µl of RT Buffer, 0.8 µl of dNTP, 1 µl of reverse transcriptase, 1 µl of RNase inhibitor, 2 µl of RT random primers, and then the total volume was completed to 20 µl using nuclease-free water.
Following reverse transcription, cDNA was stored at –20°C to be used in real-time quantitative polymerase chain reaction (RT-qPCR) experiments. Thermo Scientific Maxima SYBR Green qPCR Master Mix (2X) kit (Thermo Fisher Scientific Inc., Cat. No. K0251) was used to perform RT-qPCR. For amplification of the lncRNA HOTAIR gene, the following sequences of forward and reverse primers were used: forward primer (HOTAIR-F), 5’-GGTAGAAAAAGCAACCACGAAGC-3’ and reverse primer (HOTAIR-R), 5’-ACATAAACCTCTGTCTGTGAGTGCC-3’. For amplification of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene as an endogenous control, the following sequences of forward and reverse primers were used: forward primer (GAPDH-F), 5’-GAAGGTGAAGGTCGGAGTCAAC-3’ and reverse primer (GAPDH-R), 5’-CAGAGTTAAAAGCAGCCCTGGT-3’. Each reaction contained 12.5 µl of Maxima SYBR Green qPCR Master Mix (2X), 1 µl of forward primer (50 pmol), 1 µl of reverse primer (50 pmol), 0.1 µl of ROX solution, 7.4 µl of nuclease-free water and 3 µl of cDNA. Samples were assayed in duplicate. RT-qPCR was programmed as follows: an initial activation step at 95°C for 10 minutes, followed by 3-step cycling (40 cycles): denaturation step at 95°C for 15 seconds, annealing step for 30 seconds at 63°C for lncRNA HOTAIR gene and 65°C for GAPDH gene, and finally an extension step at 72°C for 30 seconds.
Melting curve analysis was performed to verify specificity and identity of PCR products. Melting curve analysis was built into the software of real-time cyclers. Generally, melting curve data were analyzed between 59°C and 95°C. When the temperature was gradually increased, a sharp decrease in SYBR Green fluorescence was observed as the product underwent denaturation. Specific products were distinguished from nonspecific products by the difference in their melting temperatures. To avoid misinterpretation, negative controls were included in all runs. A blank control without DNA was included in each PCR run as a negative control. In order to avoid mispriming or other errors that might happen during PCR, Hot-Start PCR was used. Results were analyzed using StepOne Software v2.2. The fold change between a sample and a normal control for lncRNA HOTAIR was calculated with the relative quantification method (RQ = 2–ΔΔCT).
Statistical analysis
Data were fed to the computer and analyzed using the software SPSS Statistics version 20.0. (SPSS, Armonk, NY: IBM Corporation). Qualitative data were described as number and percentage. Quantitative data were described as range and mean ± standard deviation. The Kolmogorov-Smirnov test and Shapiro-Wilk test were used to verify the normality of data distribution. The MannWhitney U-test was used to compare between the groups for non-normally distributed numerical variables. Student’s t-test was used to compare between the groups for normally distributed numerical variables. The receiver operating characteristic (ROC) curve was plotted to determine the optimal cut-off value of the tested variables with the highest sensitivity and specificity for prediction of the outcome. The area under the curve (AUC), the positive predictive value (PPV) and the negative predictive value (NPV) were calculated for this cut-off value. Statistical significance of the obtained results was judged at the p < 0.05 level. All calculated p values were two-tailed.
Results
Baseline clinical and biochemical data of patients and healthy subjects included in the study are shown in Table 1.
Table 1
Distribution of clinical and biochemical data of patients and healthy subjects included in the study
Serum AFP level
The mean serum AFP level was significantly higher in groups IA, IB and II compared to group III (p < 0.001). The mean serum AFP level was also significantly higher in group IA than groups IB and II (p = 0.003 and p = 0.001 respectively), but showed no significant difference between group IB and group II (p = 0.096) (Table 2).
Table 2
Statistical comparison between the groups of patients included in the study and healthy subjects as regard α-fetoprotein (AFP) serum level (ng/ml)
[i] AFP – α-fetoprotein, HCC – hepatocellular carcinoma, U – Mann-Whitney test, p – value of comparison, Pcontrol – p value for comparing between control and each other group, p1 – p value for comparing between late and early HCC cases, p2 – p value for comparing between late HCC and cirrhosis, p3 – p value for comparing between early HCC and cirrhosis, *statistical significance at p ≤ 0.05
Serum HOTAIR expression
The mean serum HOTAIR expression was significantly higher in groups IA, IB and II compared to group III (p < 0.001). The mean serum HOTAIR expression was significantly higher in group IA than group IB (p = 0.013), and also was significantly higher in groups IA and 1B than group II (p < 0.001 and p = 0.018 respectively) (Table 3).
Table 3
Statistical comparison between the groups of patients included in the study and healthy subjects as regard 2–ΔΔCt HOTAIR serum level
[i] HOTAIR – homeobox transcript antisense intergenic RNA, HCC – hepatocellular carcinoma, U – Mann-Whitney test, p – value of comparison; pcontrol – p value for comparing between control and each other group, p1 – p value for comparing between late and early HCC cases, p2 – p value for comparing between late HCC and cirrhosis, p3 – p value for comparing between early HCC and cirrhosis, *statistical significance at p ≤ 0.05
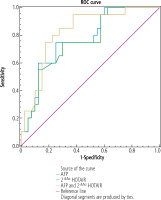
Performance of serum HOTAIR expression to discriminate between HCC patients of BCLC stages 0/A-B and HCC patients of BCLC stages C-D
Serum AFP at cut-off value > 50 ng/ml (AUC = 0.73, p = 0.003) showed sensitivity of 60% and specificity of 80% in discriminating HCC patients of group IB from HCC patients of group IA. Serum HOTAIR at cut-off value > 15.45 (AUC = 0.71, p = 0.013) showed sensitivity of 66% and specificity of 78% in discriminating HCC patients of group IB from HCC patients of group IA. When serum HOTAIR was combined with AFP, the discriminative sensitivity and specificity increased to 74% and 90% respectively (AUC = 0.85, p < 0.001) (Table 4 and Fig. 1).
Table 4
Performance of circulating HOTAIR solely and combined with AFP to discriminate between HCC patients of BCLC stages 0/A-B and HCC patients of BCLC stages C-D
Performance of serum HOTAIR expression to discriminate between HCC patients of BCLC stages 0/A-B and patients with non-tumorous liver cirrhosis
Serum AFP at cut-off value > 150 ng/ml (AUC = 0.882, p < 0.001) showed sensitivity of 65% and specificity of 100% in discriminating HCC patients of group IB from patients with non-tumorous liver cirrhosis. Serum HOTAIR at cut-off value > 9.42 (AUC = 0.823, p < 0.001) showed sensitivity of 67.5% and specificity of 93.3% in discriminating HCC patients of group IB from patients with non-tumorous liver cirrhosis. When serum HOTAIR was combined with AFP, the discriminative sensitivity and specificity increased to 80% and 98.3% respectively (AUC = 0.954, p < 0.001) (Table 5 and Fig. 2).
Fig. 2
Performance of circulating HOTAIR solely and combined with AFP to discriminate between HCC patients of BCLC stages 0/A-B and patients with non-tumorous liver cirrhosis
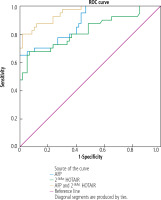
Table 5
Performance of circulating HOTAIR solely and combined with AFP to discriminate between HCC patients of BCLC stages 0/A-B and patients with non-tumorous liver cirrhosis
Discussion
LncRNA HOTAIR has been increasingly recognized as a major player in diverse cellular functions, and found to interact by epigenetic mechanisms to affect the chromatin state and regulate target genes [11, 16]. However, the role of HOTAIR in liver fibrosis remains unknown. The current study found that serum HOTAIR expression was significantly higher in cirrhotic patients compared to the healthy subjects. Similarly, HOTAIR expression was significantly increased in human fibrotic livers and activated hepatic stellate cells (HSC) through regulation of different physiological and pathological processes [23-25]. Moreover, HOTAIR expression level was increased in cirrhotic liver tissues from hepatitis B virus-infected patients and co-localized with smooth muscle actin alpha 2 (ACTA2), indicating that HSC may be the primary source for HOTAIR in fibrotic liver [23]. A functional characterization demonstrated that HOTAIR overexpression promoted cell proliferation and activation with up-regulation of fibrosis-related genes and downstream mediators, such as ACTA2, collagen type 1 alpha 1 (COL1A1), and matrix metalloproteinases (MMP) [23, 24]. Furthermore, HOTAIR may act as an endogenous ‘sponge’ of certain micro-RNA (miR) molecules, such as miR-148b and miR-29b, which regulate the expression of various signaling pathways involved in HSC activation and proliferation [23, 25]. These results suggest that HOTAIR suppression may represent a possible therapeutic target in liver fibrosis.
HOTAIR has been extensively studied as an oncogene in numerous cancers including HCC [9, 16, 19]. The current study found that serum HOTAIR was clearly over-expressed in patients with HCC compared to cirrhotic patients and healthy subjects, and the increase of serum HOTAIR was compatible with the advancement of HCC stage. Similarly, HOTAIR was significantly over-expressed in HCC compared to the adjacent non-cancerous cirrhotic liver tissues, and HOTAIR depletion significantly inhibited proliferation and invasion of liver cancer cells in vivo and in vitro [26-28]. Those findings suggested that HCC proliferation may be affected by HOTAIR expression in the cells. Likewise, HOTAIR was over-expressed in HCC as compared with the adjacent non-tumorous cirrhotic liver tissues and was associated with tumor size [29]. Recently, a study showed significantly higher expression of HOTAIR in tumor tissues compared to adjacent tissues and in peripheral blood of HCC patients compared to healthy controls, with a positive correlation between its expression in the tumor tissue and peripheral blood [30]. Moreover, HOTAIR was up-regulated in non-cirrhotic CLD patients, cirrhotic patients and HCC patients, and the level of increase was consistent with the progression of the disease toward HCC, and this was associated with advanced stages and higher grades of the tumor [31]. Although HOTAIR did not discriminate between cirrhotic cases and non-cirrhotic CLD cases, it showed significant capability to discriminate between cases of cirrhosis and HCC compared with healthy controls [31]. That study suggested that HOTAIR could be considered as a predictor and prognostic biomarker and a new therapeutic target for HCV-induced cirrhosis and HCC [31]. Intriguingly, HOTAIR expression significantly predicted the development of HCC in HCV-infected patients treated with direct-acting antiviral therapy, and the higher values correlated with poorer disease outcomes [32]. That study suggested that pre-treatment HOTAIR expression level could serve as a risk assessment biomarker for HCC in HCV-infected patients and should be rigorously taken into consideration [32].
The current study demonstrated that HOTAIR may potentially differentiate early-stage HCC from late-stage HCC. Likewise, HCC patients with higher HOTAIR expression in their tumors have shown an increased risk of lymph node metastasis and recurrence after hepatectomy [27]. Also, HOTAIR expression increased in both HCC and HepG2 cells, promoted tumor progression and was associated with earlier relapse [28, 29, 33]. Those results highlighted the importance of HOTAIR in the evolvement and the relapse of HCC. Intriguingly, the expression level of HOTAIR in HCC was significantly associated with poor tumor differentiation and early recurrence, whereas no associations of HOTAIR expression with cirrhosis, serum AFP level, and TNM stage of the tumor were revealed [28]. Notably, a high HOTAIR expression level was found to be an independent prognostic factor for predicting HCC recurrence in transplanted patients and shorter recurrence-free survival [26]. Also, it was found that HCC patients with low HOTAIR expression in tumor tissues and/or peripheral blood had significantly longer overall and progression-free survival and a favorable response to therapy in patients with advanced HCC [30].
Various mechanisms have been suggested to understand the oncogenic functions of HOTAIR gene expression in regulating HCC progression [34, 35]. Up-regulation of HOTAIR promoted the proliferation, migration, and invasion of human HCC cells through inducing certain autophagy-related genes (ATG), e.g. ATG3 and ATG7, which are indispensable for autophagosome formation and activation [29, 33, 36]. Also, HOTAIR plays a critical role in the progression of HCC via inhibition of RNA binding motif protein 38 (RBM-38) [37]. Moreover, in vitro assays in the HCC cell line Bel7402 demonstrated that knockdown of HOTAIR reduced cell proliferation and was associated with reduction in the levels of vascular endothelial growth factor (VEGF) protein and MMP-9, which are important for cell motility, tumor progression and metastasis [27]. Furthermore, HOTAIR reduces the expression of Wnt inhibitory factor 1 (WIF-1), causing activation of Wnt/β-catenin signaling, which indicates that HOTAIR is important in the progression and recurrence of HCC, partly through the regulation of this molecular mechanism [28, 38]. Additionally, in vitro and in vivo experiments of HOTAIR knockdown induced apoptosis, inhibited cell proliferation and migration, impeded the epithelial-mesenchymal transition, and finally reduced the metastatic potential of HCC [39]. Epigenetically, HOTAIR may sponge miR-217-5p and suppress miR-122 expression via DNA methylation, leading to promotion of tumorigenicity and cell progression in HCC [40, 41].
Conclusions
In the current study, HCC was clearly accompanied by over-expression of circulating HOTAIR and the level of this expression was significantly associated with tumor stage and progression. These results suggest that the circulating HOTAIR test may be used solely, or preferably in combination with the serum AFP test, to aid in HCC detection and staging. Hence, HOTAIR is a valuable clinical biomarker for HCC diagnosis and prediction of the clinical outcome. Also, it may help monitoring the response to treatment and detection of tumor relapse. Targeting HOTAIR may be a novel therapeutic target for precision therapy in HCC.
In order to correct for the limitations in the present work, it may be recommended that the validity of circulating HOTAIR expression to diagnose HCC in cirrhotic liver and differentiate the tumor stage should be extensively studied in prospective longitudinal studies of a large population including patients with other etiologies of CLD. Moreover, evaluation of HOTAIR expression in HCC tissue and peri-tumor cirrhotic liver tissue paired with blood expression in patients with different HCC grades and tumor stages would certainly help to fully explore its potential contribution to liver cancer progression and prognosis. Furthermore, it is imperative to assess its clinical value as a biomarker to help monitoring the response of HCC to treatment and detection of the tumor recurrence after surgical, locoregional or systemic therapies. Further studies to search for any significant role of HOTAIR expression in the pathophysiology of different stages of liver disease should be conducted as well.