Introduction
Asthma is a common and serious chronic disease that affects an estimated 358 million individuals worldwide [1]. Depending on the definitions and health-care settings, it is estimated that 5% to 10% of the whole population with asthma suffers from severe refractory asthma, which requires maximum recommended treatment with combinations of anti-inflammatory and bronchodilator drugs [2]. According to the Global Initiative for Asthma (GINA) guidelines, severe asthma is defined as a disease that remains uncontrolled despite adherence to optimized therapy (GINA step 4 or 5 treatment) and treatment of contributory factors or that requires such treatment for good symptom control and reduction of the risk of exacerbations [1]. The disease represents a significant burden on both the patients’ budgets and financial resources allocated to health care due to direct costs (e.g. frequent hospitalizations, emergency room visits, expensive intensive treatment) and indirect costs (e.g. lost productivity) [3, 4].
As asthma is a heterogeneous disease, identifying distinct clinical phenotypes as well as underlying immune molecular mechanisms (endotypes) is important in selecting the appropriate treatment. In line with the GINA guidelines, severe asthma management depends on the type of inflammation involved, namely the presence of T helper 2 (Th2)-high or Th2-low endotypes [1]. Th2-high is often associated with eosinophilia, increased fraction of exhaled nitric oxide (FENO), and atopy, with cytokines such as interleukin (IL)-4, IL-5, IL-13, and class E immunoglobulin (IgE) playing a key role. This endotype is dominant in early-onset allergic and late-onset eosinophilic asthma. In the case of Th2-low endotypes, found in neutrophilic asthma and obesity-related asthma, Th1 and Th17 immunity as well as neutrophilic inflammation are involved [5].
Monoclonal antibodies have become a milestone in more personalized and precise treatment of some phenotypes of severe asthma. In Poland, only biologics targeting the key mechanisms related to Th2-predominant inflammation are available to patients covered by the National Programme for the Treatment of Severe Asthma and are fully reimbursed by the health service [6]. Omalizumab (OMA) was approved as a drug supporting optimized treatment for severe allergic asthma in Poland on 17 March 2013 [7]. It is a recombinant humanized monoclonal antibody directed against IgE. The observational study evaluating the effectiveness of the Polish OMA programme showed significant benefits for patients, including reduced use of oral corticosteroids (OCS), improved asthma control and quality of life [7, 8]. Mepolizumab (MEPO) (anti-IL-5) and benralizumab (BENRA) (anti-ILR5) have been available as a treatment for severe eosinophilic asthma since 1 November 2017 and 1 November 2019, respectively [9]. Numerous studies have confirmed the efficacy of these drugs in reducing the number of exacerbations and OCS use, improving quality of life and airflow parameters [10–13]. It is worth mentioning that the new GINA guidelines also recommend other biologics such as dupilumab and tezepelumab. A multitude of therapies available targeting different signalling pathways highlight the key element of phenotyping in achieving optimal clinical effect.
It is estimated that in Poland 15,000 individuals with severe asthma are candidates for biological treatment, while currently only about 1,100 are included in the therapeutic programme [9]. Correct classification of patients eligible (EL) for biologics may be difficult outside of specialist asthma treatment centres that have access to diagnostic tools and expert knowledge. Current limitations include access to epidemiological data on severe asthma from patients treated in these centres, which justifies the extension of research in this area.
Aim
The primary aim of the study was to identify difficulties in the qualification process and to establish predominant reasons leading to therapeutic programme exclusions. The secondary aim was to determine the clinical profile of EL and ineligible (InEL) patients referred for biological therapy. We hope that this knowledge will contribute to the improvement of the qualification process and allow more severe asthmatics to access biological therapy.
Material and methods
Study design
The project was designed as an observational, retrospective and single-centre study including historical data from one visit of a patient referred for qualification for biological therapy of Th2-high asthma phenotypes (allergic or eosinophilic or allergic-eosinophilic overlap asthma) in 2018/2019 to the Barlicki Hospital (Lodz, Poland). During the visit, eligibility and ineligibility of the patients had been determined on the basis of criteria defined by the Polish National Health Fund (NHF) [14]. If the criteria for severe asthma were not met, the need to verify the diagnosis had been noted in the medical records.
For the purpose of the study, data from the said visit as well as medical history from hospital records were acquired. For each individual the following information was used: demographic data, smoking status, asthma control, exacerbations requiring short courses of systemic corticosteroids (or temporary increase in basal OCS dose) for at least 3 days, pharmacotherapy, mean daily dose of OCS over the last 6 months, number of hospitalization due to asthma, complications induced by systemic steroid therapy, blood eosinophil count (EOScount), skin prick test (SPT) or allergen-specific IgE (sIgE), serum total IgE (tIgE), comorbidities and associated medical treatment. Forced spirometry had been performed according to the European Respiratory Society and American Thoracic Society (ERS/ATS) standards during the qualification visit. The analysis had included the following parameters: forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) reported in litres, and percentages of predicted values. The FEV1%FVC index (FEV1 and FVC quotient) had been expressed in absolute numbers. Quality of life had been assessed using the self-administered Asthma Quality of Life Questionnaire (AQLQ) and asthma control by the Asthma Control Questionnaire (ACQ). In addition, the factors determining the ineligibility for the biological treatment described by a physician from a specialist centre were included in the study.
Statistical analysis
Statistical analysis was performed using methods of descriptive statistics. The comparisons between groups of patients EL and InEL for biological therapy were analysed for continuous variables using the t-test for independent samples, the Mann-Whitney U-test in case of failure to meet the assumptions of the parametric test, and the χ2 test for discrete variables. The p-value < 0.05 was considered statistically significant. Statistical analysis of the data was performed using Statistica™ (TIBCO Software Inc. 2017).
Results
Demographic
In total, 116 adult patients (mean age 52 years, 65% women) were included in this study. Subjects had been diagnosed and managed according to the routine requirements of clinical practice and the programme criteria. Most asthma patients (n = 93, 8%) had been qualified to be treated with biological therapy. Among the EL patients, the greatest number had been included in the MEPO treatment programme, followed by OMA and BENRA therapy. More than a third of the overall study subjects had had late onset asthma (asthma diagnosis over the age of 40) (37.9%). Only 1 patient from the InEL group had been smoking regularly (Table 1).
Table 1
Patient demographic data
[i] BMI – body mass index, M – mean, SD – standard deviation, y.o. – years old. P-value was calculated for comparisons between eligible and ineligible groups. Percentages in brackets has been calculated based on the number of subjects in the study (column 1), ineligible patients (column 2) or eligible patients (column 3).
Atopy, comorbidities, and biomarkers
The most common atopic disease had been allergic rhinitis which had been diagnosed most often among patients with atopy (n = 68 of 72 total). Allergies had been confirmed by SPT or sIgE test and had revealed that the house dust mite had been the most common perennial allergen (n = 46 of 72 total, 64%). Comorbidities were highly prevalent in both groups (EL and InEL). The most common comorbidities had been allergic rhinitis (58.6%), then: chronic rhinosinusitis (41%) obesity (34.5%), and gastroesophageal reflux disease (25%). Analysis showed that EOScount was significantly higher in the EL group (M = 658/μl) compared with the InEL group (M = 295/μl) (Table 2).
Table 2
Comorbidities, atopy and biomarkers
| Variables | Overall (n = 116) | Ineligible (n = 23) | Eligible (n = 93) | P-value |
|---|---|---|---|---|
| Atopy, n (%) | 72 (62.1) | 12 (52.2) | 60 (64.5) | 0.275 |
| Allergy, n (%)*: | ||||
| Dust mites | 46 (63.9) | 4 (44.4) | 42 (66.7) | 0.194 |
| Moulds | 19 (26.4) | 1 (11.1) | 18 (28.6) | 0.266 |
| Cat | 21 (29.2) | 2 (22.2) | 19 (30.2) | 0.624 |
| Dog | 22 (30.6) | 2 (22.2) | 20 (31.7) | 0.562 |
| Serum tIgE [IU/ml], M (SD) | 341.2 (430.4) | 178.3 (363.3) | 364.005 (437.3) | 0.289 |
| EOS [/μl]**, M (SD) | 581 (459) | 295 (287) | 658 (467) | < 0.001 |
| Allergic rhinitis, n (%) | 68 (58.6) | 11 (47.8) | 57 (61.3) | 0.240 |
| Food hypersensitivity, n (%) | 2 (1.8) | 0 (0.0) | 2 (2.2) | 0.471 |
| Atopic dermatitis, n (%) | 3 (2.6) | 0 (0.0) | 3 (3.2) | 0.383 |
| Chronic sinusitis, n (%) | 48 (41.4) | 7 (30.4) | 41 (44.1) | 0.234 |
| Nasal polyps, n (%) | 35 (30.2) | 4 (17.4) | 31 (33.3) | 0.136 |
| Polypectomy, n (%) | 29 (25.0) | 3 (13.0) | 26 (28.0) | 0.139 |
| GERD, n (%) | 29 (25.2) | 4 (17.4) | 25 (27.2) | 0.334 |
| NSAID sensitivity, n (%) | 25 (21.7) | 4 (17.4) | 21 (22.8) | 0.527 |
| ACOS, n (%) | 11 (14.0) | 2 (8.7) | 9 (10.6) | 0.790 |
| Depression, n (%) | 11 (9.5) | 3 (13.0) | 8 (8.6) | 0.515 |
tIgE – total immunoglobulin E levels, EOS – eosinophil blood count (absolute), GERD – gastroesophageal reflux disease, NSAID – non-steroidal anti-inflammatory drug, ACOS – asthma-COPD overlap syndrome, M – mean, SD – standard deviation. P-value was calculated for comparisons between eligible and ineligible groups. Percentages in brackets has been calculated based on the number of subjects in the study (column 1), ineligible patients (column 2) or eligible patients (column 3).
Asthma control, treatment, and exacerbations
All patients had received high doses of inhaled corticosteroids (ICS) and Long-Acting Beta Agonists (LABA) treatment. The most common add-on to ICS and LABA had been leukotriene receptor antagonists (LTRA) (65%) followed by short/long-acting muscarinic receptor antagonists (SAMA/LAMA) (45%) and theophylline (15%). EL patients were significantly more likely to be receiving LTRA and single inhaler dual therapy (LABA + ICS) than InEL patients. There were no significant differences between the EL/InEL group in the number of exacerbations, asthma treatment regimen, quality of life, or asthma control (Table 3).
Table 3
Asthma control, treatment and exacerbations
| Variables | Overall (n = 116) | Ineligible (n = 23) | Eligible (n = 93) | P-value |
|---|---|---|---|---|
| Exacerbations requiring short courses of OCS*[/year], M (SD) | 3.3 (1.8) | 2.857 (2.1) | 3.441 (1.7) | 0.070 |
| Hospitalizations in the preceding year, M (SD) | 0.8 (0.8) | 0.8 (0.9) | 0.8 (0.8) | 0.963 |
| Life-threatening asthma events, n (%) | 8 (7.4) | 1 (5.0) | 7 (8.0) | 0.649 |
| ACQ score, M (SD) | 3.3 (0.9) | 3.0 (0.9) | 3.4 (0.9) | 0.080 |
| AQLQ score, M (SD) | 3.3 (1.0) | 3.3 (1.0) | 3.3 (1.0) | 0.924 |
| OCS maintenance**, n (%) | 49 (43.4) | 9 (39.1) | 40 (44.4) | 0.646 |
| OCS dose [mg/day]***, M (SD) | 7.6 (6,7) | 5.2 (4,0) | 8.1 (7.0) | 0.108 |
| BDP-CFC ICS equivalent dose [mg/day], M (SD) | 2933.0 (1149.7) | 2544.8 (1049.8) | 3020.6 (1158.3) | 0.122 |
| Complications after OCS, n (%): | ||||
| Arterial hypertension | 51 (44.0) | 11 (47.8) | 40 (43.0) | 0.677 |
| Dyslipidaemia | 25 (21.6) | 2 (8.7) | 23 (24.7) | 0.094 |
| Obesity (> 30 kg/m2) | 40 (34.5) | 6 (26.1) | 34 (36.6) | 0.344 |
| Cataract | 4 (3.4) | 0 (0.0) | 4 (4.3) | 0.311 |
| Glaucoma | 5 (4.3) | 0 (0.0) | 5 (5.4) | 0.256 |
| Diabetes | 20 (17.2) | 3 (13.0) | 17 (18.3) | 0.522 |
| Asthma treatment: | ||||
| ICS, n (%) | 116 (100.0) | 23 (100.0) | 93 (100.0) | 1.000 |
| ICS + LABA****, n (%) | 90 (77.6) | 14 (60.9) | 76 (81.7) | 0.032 |
| SABA, n (%) | 113 (98.3) | 21 (95.5) | 92 (98.9) | 0.263 |
| LABA, n (%) | 116 (100.0) | 23 (100.0) | 93 (100.0) | 1.000 |
| SAMA/LAMA, n (%) | 52 (45.2) | 10 (45.5) | 42 (45.2) | 0.980 |
| LTRA, n (%) | 75 (65.2) | 10 (45.5) | 65 (69.9) | 0.030 |
| Theophylline, n (%) | 17 (14.8) | 3 (13.6) | 14 (15.1) | 0.866 |
| SABA use per day [dose], M (SD) | 4.3 (2.9) | 3.2 (2.3) | 4.6 (3.0) | 0.061 |
ACQ – asthma control questionnaire, AQLQ – asthma quality of life questionnaire, BDP-CFC – inhaled beclomethasone CFC, ICS – inhaled corticosteroid, LABA – long-acting α-adrenoceptor agonist, LAMA – long-acting muscarinic antagonist, LTRA – leukotriene receptor antagonists, OCS – oral corticosteroid, SABA – short-acting α-adrenoceptor agonist, SAMA – short-acting muscarinic antagonist, M – mean, SD – standard deviation. P-value was calculated for comparisons between eligible and ineligible groups. Percentages in brackets have been calculated based on the number of subjects in the study (column 1), ineligible patients (column 2), or eligible patients (column 3).
In addition, applying the EOScount cut-off ≥ 350/μl, 28.2% of patients eligible for OMA therapy had overlapped with eosinophilic asthma. In contrast, 40.4% of patients qualified to receive MEPO therapy had exhibited features of allergic asthma.
Respiratory function
Overall the analysis showed a number of differences in airflow parameters between EL/InEL groups. FEV1 was significantly higher and less obstructive in the InEL group. Surprisingly, we found that 43% (n = 10 of 23 total) and 56.5% (n = 13 of 23 total) of InEL patients had had FEV1 > 80% and FEV1/FVC > 70%, respectively (Table 4).
Table 4
Respiratory function
[i] FEV1 – forced expiratory volume in 1 s, FVC – forced vital capacity, M – mean, SD – standard deviation. P-value was calculated for comparisons between eligible and ineligible groups. Percentages in brackets has been calculated based on the number of subjects in the study (column 1), ineligible patients (column 2) or eligible patients (column 3).
Characteristics of InEL patients for biological therapy
A total of 23 patients had been ineligible for biological therapy (9 for OMA, 9 for MEPO, for 5 patients, an attempt had been made to qualify for any of the treatment).
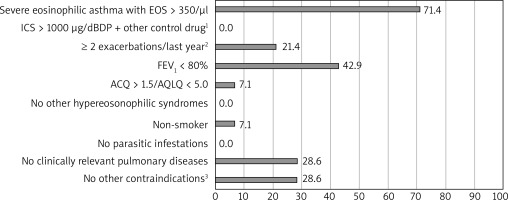
Based on the OMA programme the most demanding criteria to meet had been: life-threatening asthma in the past, FEV1 < 60%, serologic criterion (tIgE), and frequent use of OCS in the past (respectively). For MEPO the least frequently fulfilled criteria were EOScount > 350/μl and FEV1 < 80%. The percentage of patients who had not met each particular criterion is shown in Figures 1 and 2.
Figure 1
Percentage of study ineligible participants who did not meet major and minor criteria for omalizumab therapy; n = 14. A minor criterion of six minor criteria; at least three of six criteria have to be fulfilled in order to qualify the patients for the programme: 1) confirmed by skin prick tests or specific IgE tests, 2) other control drugs: long-acting β-adrenoceptor agonists, leukotriene receptor antagonist, theophylline, 3) confirmed in vitro reactivity (RAST) to perennial allergens in the case of patients with total IgE serum concentration below 76 U/ml, 4) requiring the use of systemic corticosteroids, 5) other than an allergic reaction to perennial inhalant allergens
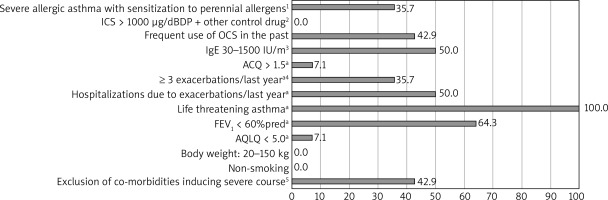
Figure 2
Percentage of study ineligible participants who did not meet major criteria for mepolizumab therapy; n = 14. 1) Other control drugs: long-acting β-adrenoceptor agonists, leukotriene receptor antagonists, theophylline, 2) requiring the use of systemic corticosteroids, 3) contraindications: simultaneous therapy with immunosuppressive drugs, anticancer drugs, immunoglobulin infusions or other biological therapies
Discussion
According to the Lodz epidemiological data [15] and estimated prevalence of severe asthma [2], around 2700 severe-asthma patients live in this region. However, in the Lodz specialist centre, which is one of the largest in Poland [7], only 93 people had qualified for biological treatment within 2 years. In this study, which is the first to present data on the detailed qualification process and the clinical profile of EL and InEL patients, we propose an explanation for potential causes of this disproportion by identifying difficulties and limitations in qualifying patients for the NHF programme.
We observed that during the qualification process, the least frequently fulfilled major criteria for OMA had been the serologic criterion (tIgE) and frequent use of OCS in the past, including in the last 6 months. Moreover, among the minor criteria, nobody had met the life-threatening asthma point, and only approximately one-third had met the spirometric criterion. For MEPO the least frequently fulfilled criteria had been EOScount > 350/μl and FEV1 < 80%. One-third of patients had had contraindications to therapy (anticancer therapy or another biological therapy – dupilumab). 42.9% and 28.6% of patients in the InEL group had not been enrolled in the OMA and MEPO programme, respectively, due to the possibility of incorrect diagnosis of severe asthma. They had been referred to re-verify the diagnosis of severe asthma.
A comparison of our results with available studies shows that the programme’s eligibility criteria were more restrictive in Poland and therefore characteristics of the Polish cohort indicate a more severe course of asthma in EL patients than in other populations [16–18]. Not surprisingly, in our study, the least frequently fulfilled criterion for MEPO therapy was EOScount > 350/μl. A similar phenomenon was observed by Richards et al. who demonstrated that 41.2% of the mepolizumab-receiving patients with severe asthma would have been ineligible because of EOScount < 300/ μl [19]. Interestingly, a secondary analysis of the DREAM and MENSA studies have revealed clinically relevant reductions in exacerbation frequency in patients with a count of 150/μl or more at baseline [20]. In the light of this research and ERS/ATS recommendation [21], the EOScount > 350/μl criterion in the Polish MEPO programme seems to be too restrictive, especially in the group of patients using prolonged OCS treatment. According to the Birmingham Regional Severe Asthma Centre (BRSAS) registry, most of the respondents did not meet the spirometric criterion FEV1 < 80% (39.7%), then atopy (29%) (based on the National Institute of Health and Care Excellence criteria) [22]. These findings are similar to our results in regard to OMA least frequently fulfilled criteria [23].
As shown in cluster analysis, patients with “symptom-predominant phenotypes” have the multifactorial aetiology of symptoms and may not be directly related to underlying eosinophilic airway inflammation [24]. In this subgroup, careful diagnosis for comorbidities that may worsen the course of asthma, in particular bronchial hyperreactivity or bronchiectasis, is recommended. Buhl et al. found that patients with uncontrolled asthma are often not referred to asthma specialist care [25]. This was in line with our study, which demonstrated that a high percentage of patients are still referred to biological therapy without previous accurate differentiation between difficult-to-treat asthma and severe asthma and with incorrect intensification of asthma therapy. In our cohort, we noticed that despite less EOScount and better spirometric parameters in the InEL group there were no significant differences between EL/InEL in the number of exacerbations, asthma treatment regimen, quality of life, or asthma control, which demonstrates that patients not eligible for the programme should receive specialist care and continuous effort to establish alternative treatment.
Interestingly, we have noticed that patients in this study had been diagnosed with severe asthma several years prior to referral to the specialist asthma clinic (Table 1). Moreover, 28% of patients had been referred for possible qualification with significant contraindications to biological therapy. These findings suggest still low awareness and knowledge among physicians about the most current treatment options.
The low awareness and knowledge of physicians regarding the qualifications for biological treatment as well as relatively frequent errors in the diagnosis of severe asthma indicate the unmet need to optimize the model of healthcare organization in Poland. In our opinion, organizing severe asthma treatment at the national level, improving access to specialized asthma centres, but also implementing information technology (IT) solutions using the e-Prescription system support disease management in accordance with the GINA guidelines may be of key importance in optimizing severe asthma care in the future [26]. At the same time, the efforts of experts should focus on optimizing the inclusion/exclusion criteria for biological treatment so that it is accessible to a larger number of patients. Indeed, thanks to numerous discussions and suggestions of physicians qualifying patients for biological treatment, on 1 May 2022, the requirements of the Severe Asthma Treatment Programme in Poland were changed. Specifically, in the case of three drugs currently available in the Programme, the inclusion criteria have been standardized. There was also a record reducing the required level of eosinophilia (to a minimum of 150 cells/μl) in patients qualified for treatment with MEPO and BENRA during chronic systemic steroid therapy. From now on, there are no contraindications for biological treatment with simultaneous therapy with immunosuppressants, anti-cancer drugs, infusions of immunoglobulins, or other biological drugs. In our opinion, less restrictive criteria will have a positive impact on the therapeutic management of patients with severe asthma.
All patients had received treatment at GINA step 5 [1]. In our report, the asthma treatment pattern was similar to the large Belgian [16] and the International Severe Asthma Registry (ISAR) [17]. However, the mean ICS dose converted to chlorofluorocarbon (CFC)-beclomethasone dipropionate equivalent was significantly higher than observed in other severe asthma populations [16, 18]. In our study, almost half of the patients had required systemic corticosteroid maintenance therapy in the previous year before the qualification process. However, as shown by the Severe Asthma Research Programme (SARP), the percentage of OCS treatment may vary between countries and depend on the different OCS dosing regimens observed in Europe [27]. We also found that the proportion of late-onset asthma patients had been higher (37.9%) than observed in the Belgian cohort (31%) [16] and the ISAR registry (34.4%) [17]. The high prevalence of the late-onset disease in our cohort supports the observation that this phenotype plays a key role in severe asthma [28].
The mean number of EOScount in our cohort (581/μl) was much higher than that observed in most of the studies [16–18]. The association between EOScount and risk of severe exacerbations, poorer lung function and loss of asthma control is well documented [29, 30]. In our study, overall patients with EOScount greater than 400/μl (61/109, 56%) compared with patients with EOScount < 400/μl (48/109, 44%) had had poorer asthma control using ACQ (3.44 vs. 3.03, p = 0.029) and lower spirometric parameters: FEV1%pred. (59.8% vs. 70.05%, p = 0.012) and FEV1/FVC ratio (%) (59 vs. 66, p = 0.004). However, the correlation observed in a UK cohort study [30] between greater EOScount and a higher risk of severe exacerbations compared to the group with EOScount < 400/μl was not confirmed in our study.
Recent research draws attention to the phenomenon of biological treatment overlapping [23, 31]. In our study, 28.2% of patients qualified to receive OMA treatment had also met the criteria for the diagnosis of severe eosinophilic asthma and 40.4% of patients qualified to receive MEPO therapy had had at least one positive result on SPT or elevated sIgE levels to perennial allergens. When the asthma phenotypes overlap in some cases, it remains an open question which of these drugs will be more successful or will be selectively more effective in some Th2 phenotypes. Hence, a physician referring to a biological treatment clinic should accurately describe the course of asthma, treatment, number and severity of exacerbations, use of systemic corticosteroids, chronic and coexisting diseases in the patient’s medical history to facilitate the process of selecting the appropriate and more personalized therapy.
Conclusions
In light of the predominant reasons leading to therapeutic programme exclusions, we found the programme qualifying criteria limiting the group of patients to the most severe cases often with a history of hospitalization due to exacerbation, life-threatening asthma attack, or frequent use of OCS. Moreover, a high percentage of patients are constantly referred to biological therapy without previous accurate differentiation between difficult-to-treat asthma and severe asthma. Our findings also suggest still existing low awareness and knowledge among physicians who often are not familiar with the qualification criteria of biologics. The referred patients, due to their multi-disease nature, polypharmacy, and heterogeneity in asthma phenotypes, constitute a significant challenge for specialists qualifying for biological therapy.
This research represents the real-life experience of the Barlicki University Hospital, which has one of the highest numbers of patients within the NHF OMA and MEPO treatment programme in Poland, making it a perfect site for the analysis. However, our study has some significant limitations due to its retrospective nature and relatively limited number of participants. However, it should be stressed that the data were collected retrospectively from only one centre, but included about 20% of all patients enrolled in the Polish programme in 2018/2019 [26]. To the best of our knowledge, the database of the characteristics of the qualification process and clinical profile of asthma patients referred to a specialist asthma centre for biological therapy is the largest available analysis in the Polish population.








