Introduction
Basal cell carcinoma (BCC) is the most common malignant tumour of the Caucasian race [1]. A few epidemiological reports confirm a steady growing tendency of their incidence among both sexes. The highest increase of 1–10% of cases per year is observed in Australia, similar data concern the United States and the European Union [2–5]. However, accurate data are not available due to the lack of bases in many countries that conduct systematic statistics of malignant skin cancer incidence, which does not allow to properly determine the actual number of patients with BCC.
Basal cell carcinomas are most often noted in adults, between 6th and 8th decade of life. Men are more likely to suffer from the disease. BCC is characterized by a large variety of clinical images [6]. The most common variety is the nodular form located on the skin of the face and neck. Typically, the lesion is a smooth, shiny nodule with the presence of widened blood vessels on the surface. The second most frequent is the superficial form, often recognized on the skin of the torso and limbs. Macroscopically, it has the form of an erythematous plaque with small erosions covered with scabs. BCC belongs to malignant cancers characterized by local growth and low metastasis ability [7]. Most often they locate in exposed parts of the head and torso, often causing significant functional and aesthetic damages. Considering the constantly growing exposure to factors predisposing to the development of skin cancer, such as UV radiation, broad access to artificial radiation sources (solariums), ubiquitous chemical carcinogens and socio-economic changes, mainly demographic ones, non-melanocytic skin cancers may take epidemic sizes in developed societies in the near future [8]. This situation requires proper preparation of the health care system, which activities should focus on the prevention and early detection of cancer lesions on the skin, effective treatment, minimizing the risk of disease recurrence, as well as rapid response to cancer recurrence. High-frequency ultrasonography (HFUS) is considered as a first-line imaging technique for evaluation of tumour depth in non-melanoma skin cancer because of its high definition and penetration that provides sharply delineated images [9, 10]. Ultrasonography examination of BCC generates a hypoechogenic, homogeneous and well-defined pattern with characteristic hyperechoic spots.
Aim
The aim of this study was an evaluation of the clinical usefulness of high-frequency ultrasonography in monitoring the effects of basal cell carcinoma treatment, particularly early detection of residual disease and post-treatment recurrences.
Material and methods
A prospective non-blind study was performed. Seventy-eight patients with suspicious lesions of BCC were enrolled in this study. Only patients that histologic evaluation of the confirmed diagnosis of BCC (70) continued the study. The lesions were evaluated dermatoscopically in each patient before the treatment, the image was taken and then ultrasonography imaging was performed (week 0). The dermatoscopic evaluation was performed with the Dermlite DL3 apparatus (3Gen LLC, Dana Port, California), dermatoscopic images were taken by combining the dermatoscope with a magnetic additional device with a camera. A 10-fold magnification of each imaging lesion was obtained using polarized or non-polarized light to achieve better visibility of dermatoscopic features. Ultrasonography was performed using a Dermascan C® USB ultrasound apparatus manufactured by Cortex Technology APS (Hadsund, Denmark). The apparatus is FDA (II/21 CFR 892.1560) certified and meets the requirements of the European Union. All measurements presented in the study were performed using a 20 MHz head (standard focus). Before the examination, the probe and the test site were covered with a small layer of gel designed for ultrasound examination. The colours of the obtained sonographic images were in the scale of greenery, which is much better distinguishable by the human eye than the scale of grey. The ultrasound examination of each lesion was performed in two perpendicular planes, horizontal and vertical. In every patient in whom basal cell carcinoma was confirmed histopathologically, the dermatoscopic and ultrasonographic observation of the scar started after the treatment (surgical treatment, ALA-PDT or imiquimod). Three control examinations were performed 4, 12 and 24 weeks after the treatment.
The following features were evaluated in sonographic images of the scars: hypoechogenic band below the entrance echo, echogenicity and structure (homoechogenic or heteroechogenic) of the scar. The purpose of scar assessment after basal cell carcinoma treatment was the search for ultrasound features of tumour recurrence or residual disease after non-radical surgery. The control of the study consisted of healthy skin from the region symmetrical to the examined skin lesion.
All patients before the study were informed about the methodology and aims of the project, they signed a form of informed consent to participation in a clinical trial. The positive opinion of the Bioethics Committee of the Medical University of Lublin was obtained for all procedures before the start of the study (KE0254/85/2015).
Results
A total of 70 basal cell carcinomas from 70 patients were evaluated in this study. The patient population met all the inclusion criteria and consisted of 43 (61.4%) females and 27 (38.6%) males of mean age of 69.04 ±12.17 (range: 33–88). The prevalence of the BCC subtypes was nodular (57.1%), superficial (25.7%), micronodular (4.3%), infiltrative (4.3%), morpheiform (1.4%) and mixed (7.2%).
All of the 40 nodular BCC lesions were removed surgically. The presence of cancer formation was observed in the margins of removed cancers in 15% (6/40), in another 25% of cases the margin of surgical removal was narrow and was < 0.2 cm (10/40). The patients were subjected to a 6-month follow-up period. During the follow-up period, the patients were examined dermatoscopically and ultrasonographically. The first dermatoscopic symptom of cancer formation was the presence of abnormal, widened blood vessels, even if they were not found in the dermatoscopic image before the surgical procedure was performed. No other residual disease-associated dermoscopic criteria (RDADC), such as the presence of pigmentation or ulcers, were observed [11]. The clinical situation was often complicated by the presence of widened blood vessels within the scar after surgery, which can be easily mistaken for vessels during residual BCC. For 4 of 6 (66%) lesions, in which histopathological examination demonstrated a positive margin after surgery, hypo or heteroechogenic, irregularly shaped focal lesions were found in the ultrasonographic examination just under the entrance echo on the first follow-up visit (4 weeks after removal of the lesion). All cases of residual disease suspicion were verified by biopsy and the presence of cancer formation was confirmed (100%). The patients were referred for a second surgical consultation. In 2 other cases of positive margins of the removed BCC, no signs of disease recurrence were observed, either in dermatoscopic or ultrasonographic examination (Figure 1). Currently, no signs of recurrence of cancer disease are observed in the patients after 24 months from the beginning of the treatment.
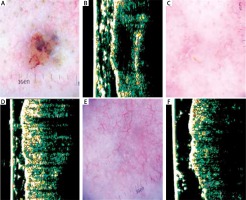
Figure 1
A – Dermatoscopy of basal cell carcinoma (BCC) of the nasal region. B – Ultrasonic image shows hypoechogenic, homogenous, well-defined structure. C – Dermatoscopy of the scar 4 weeks after surgery – in histology examination of the margin presence of cancer tissue. D – Ultrasonic image shows no evidence of cancer formation. E – Dermatoscopy of the scar 24 weeks after surgery shows multiple telangiectasia. F – Ultrasonic image 24 weeks after surgery shows no evidence of cancer formation
An increased risk of residual disease recurrence also concerns the patients with a narrow margin of healthy tissues after surgical removal. In this study, the margin of removal with an increased risk of recurrence was considered to be < 0.2 cm. The patients accounted for 1/4 of the population diagnosed with nodular BCC. In several cases, also the hypo or heteroechogenic foci located directly under the entrance echo were observed in the ultrasonographic examination 4 weeks after the surgery, suggesting the presence of cancer formation (Figure 2). Reduction in the suspected area and scar formation were observed on the subsequent visits. It was found that the characteristic feature of residual disease presence is an enlargement of the hypoechogenic area in subsequent ultrasonographic examinations (Figure 3). The presence of residual disease was confirmed histopathologically only in 1 patient (10%). In 9/10 (90%) cases of narrow, uncertain margins of surgical removal of the lesion, no recurrence was observed after 24 months of surgery. In other patients with nodular basal cell carcinoma (margins > 2 mm) no recurrence of cancer disease was observed. In the group of 18 BCC lesions of the superficial type, 15 were removed surgically and 3 were subjected to other therapeutic methods (ALA-PDT and imiquimod). The available therapeutic methods were discussed with the patients. Most patients preferred surgical treatment due to the lack of repeated visits to the Centre and low treatment costs for the patient. In no case of surgically removed lesions, the presence of cancer cells within the margin of the tumour removal, or the recurrence of cancer lesions during the study, as well as after its completion were observed. Three neoplastic lesions were subjected to 3-fold photodynamic lamp irradiation with ALA. A linear hypoechogenic area was still observed in some areas in 1 patient after therapy. Therefore, the therapy was supplemented with imiquimod used for 8 weeks. After 4 weeks from the end of treatment, normalization of the ultrasound image of the treated skin was observed, compared to the image to healthy skin. For basal cell carcinomas, ultrasonographic monitoring seems to be especially useful when applying minimally invasive therapeutic methods.
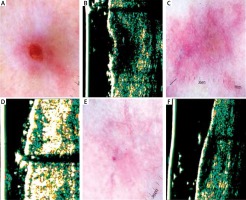
Figure 2
A – Nodular basal cell carcinoma (nBCC) of the temporal region. B – Ultrasonic image shows hypoechogenic, heterogeneous oval structure. C – Dermatoscopy of the scar 4 weeks after surgery – in histology examination, the clear surgical margin < 0.2 cm. D – Ultrasonic image 4 weeks after surgery shows hypoechogenic, not well defined structure. E – Dermatoscopy 12 weeks after surgery shows multiple telangiectasia inside the scar. F – Ultrasonic image 12 weeks after surgery reveals reduction in hypoechogenic, not well defined structure. G – Dermatoscopy 24 weeks after surgery shows telangiectasia surrounding the scar. H – Ultrasonic image 24 weeks after surgery shows no evidence of residual disease
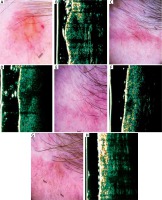
Figure 3
A – Nodular, ulcerated basal cell carcinoma of the temporal region. B – Ultrasonic image shows hypoechogenic, heterogeneous bean like structure. C – Dermatoscopy of the scar 4 weeks after surgery – in histology, the clear surgical margin. D – Ultrasonic image 4 weeks after surgery shows hypoechogenic, quite well-defined, spindle structure. E – Dermatoscopy of the scar 12 weeks after surgery shows no clear evidence of residual disease. F – Ultrasonic image 12 weeks after surgery shows enlargement of hypoechogenic, well-defined spindle structure. Biopsy confirmed residual disease
Interesting results of surgical treatment were observed in case of aggressive forms of basal cell carcinoma, which were removed surgically, in these cases, no signs of residual disease or cancer recurrence were demonstrated for 24 months after treatment (Table 1).
Table 1
Residual disease after treatment in examined basal cell carcinoma
Discussion
The indices of non-radical surgical excision of basal cell carcinoma vary depending on the study in the range of 4–16% [12]. Some attempts to define the factors related to the risk of BCC recurrence were presented in numerous reports e.g. research by Santiago et al. [13], as confirmed by observations of Farhi et al. [12]. The conclusions in both publications are consistent. They do not confirm statistically significant correlations between the positive deep margin of excision and the risk of recurrence. In both studies, significantly more frequent recurrences were observed for aggressive histopathological types of BCC and tumour localization in the vicinity of the nose and orbital cavity [13]. Sartore et al. [14] indicate a higher risk of recurrence in the presence of cancer cells in the deep margin of excision, morpheiform, metatypical or pleomorphic cancer formation, as well as cancer inflammatory infiltration. Recent studies also confirm a higher risk of recurrence in the presence of cancer cells within the deep margin of removed cancer lesions and aggressive histopathological types, as well as for recurrent lesions [15]. A multitude of factors affecting the risk of cancer recurrence, and also sometimes observed absence of cancer recurrence despite confirmed tumour cells within the margins of surgical removal (especially in the most frequently observed BCC types – superficial and nodular) significantly complicates and hinders the procedures in case of the patients after non-radical BCC removal [16]. There is no consensus on how to deal with cancer formation within the margins of surgical excision. Some authors prefer rapid re-surgery in all patients, and the follow-up strategy is recommended only in special cases where there are contraindications for re-surgery [17]. The argument confirming the correctness of this solution is the high, about 30% risk of cancer recurrence, even after 8 years of observation of the patient after the surgery, described by some researchers. However, our study clearly indicates the need to individualize the therapeutic procedure in the patients in whom the surgical excision was not radical. The residual disease in the patients with cancer formation in surgical cutting lines was confirmed in 66% (4/6) of patients and supplemental treatment was implemented, in the case of 34% patients (2/6) the lesions did not require any intervention (the observation period was 24 months). The above observations require confirmation in a larger number of patients after non-radical surgery for at least 5 years.
The dermatoscopic features of the residual disease described so far (presence of short telangiectasias and white-pink areas) do not always correspond to the presence of cancer cells in the treated area [11]. The presence of BCC dermatoscopic features (most commonly telangiectasia) and the presence of a hypoechogenic area directly under the entrance echo significantly increases the accuracy of the diagnosis of residual disease. Hernandez-Ibanez et al. [18] confirmed the usefulness of HF-USG in the diagnosis of residual disease. Undoubtedly, the limitation of this study was the small population of patients participating in the examination and the observation of only the surface variety of BCC. It seems to be a particularly important observation in our study that the areas suspected of residual disease were magnified during subsequent tests, in contrast to inflammatory lesions that decreased.
Conclusions
The above observations prove that the use of high-frequency ultrasonography in the monitoring of patients after surgery can accelerate and improve the diagnosis of residual disease. Application of this method may also prevent unnecessary re-operations. Certainly, high-frequency ultrasonography is also useful in an evaluation of the course of treatment with minimally invasive methods (ALA-PDT, imiquimod), increasing the accuracy of the decisions made, and thus optimizing the therapeutic process.








