Introduction
Syphilis is one of sexually transmitted diseases caused by the Treponema pallidum bacteria [1, 2]. Secondary syphilis is the most common clinical form of syphilis and is more common in women [3]. Secondary syphilis which is referred to as “the great imitator” due to its numerous clinical manifestations including mucocutaneous and visceral infection with systemic dissemination of treponema pallidum, re-emerged worldwide in the last decade [4]. Accordingly, the vast spectrum of its cutaneous manifestations is rising worldwide too, along with the presentation of atypical forms, especially among patients with HIV infection [5, 6]. If left untreated, most skin lesions can spontaneously resolve, and secondary syphilis tends to progress to a latent stage. But clinical manifestations can recur for up to 5 years, leading to confusion with other inflammatory dermatoses [7, 8], such as psoriasis, drug eruption, rubella, infectious mononucleosis, erythema multiforme, pityriasis or fungal infection etc., especially pityriasis rosea. Secondary syphilis can also develop into neurosyphilis even after treatment. So early and accurate diagnosis of secondary syphilis is critical [9]. T. pallidum has always been challenging to study because of its temperature sensitivity and fragility, which makes continuous inoculation and proliferation difficult. Serological examination (including nontreponemal and treponemal antibody tests) remains the mainstay of diagnosis of syphilis. However, false-negative laboratory tests may hinder the prompt diagnosis of syphilis due to the prozone phenomenon, especially more common in HIV-infected patients [10, 11]. Since the protean clinical characteristics are coupled with inconsistently reliable serologic tests, a pathological biopsy is essential and deserved to be performed for diagnosis of secondary syphilis [9, 12].
The histopathologic appearance of the great imitator also exhibits a wide spectrum, from epidermal changes and interface dermatitis to inflammatory granuloma in the dermis relevant to clinical features. But these findings are nonspecific and not always present simultaneously.
Aim
Thus, in our retrospective study, we sought to assess and determine the frequency of histopathological features characterizing secondary syphilis in skin biopsies from patients who had serologic evidence of syphilis, which features were the most common of the specimens, and to summarize the reason of misdiagnosis by comparison with clinical features.
Material and methods
A total of 129 pathological specimens from 114 patients were reviewed, with biopsy-proven secondary syphilis diagnosed between May 2010 and May 202 in Shanghai Skin Disease Hospital. All patients had serologic evidence of syphilis after skin biopsy, and ultimately serology combined with pathological and clinical characteristics were made for confirming the diagnosis.
Paraffin sections of the skin biopsy were stained with hematoxylin-eosin (HE), and the lesions were further examined under a microscope. The specimens were categorized as having or lacking the following histologic features: focal parakeratosis, epidermis hyperplasia (irregular/psoriasiform acanthosis or pseudoepitheliomatous hyperplasia), elongated rete ridges, neutrophils in epidermis, keratinocyte apoptosis, dermal papilla oedema, dermal vasodilatation, endothelial swelling, vacuolar, lichenoid, and/or interstitial patterns of inflammation, inflammatory granuloma in the dermis, lymphocytes, plasma cells, and immunohistochemical stains, if performed.
Clinical characteristics gathered included gender, age, rash morphology, site of biopsy, prebiopsy misdiagnosis and HIV status.
And then the specimens were subcategorized by the number of the above histologic features and rash morphology.
Results
In the survey, 129 pathological specimens were collected and analysed. In a single sample, it could be observed that at least one feature or at most 13 features exist concurrently (Table 1). Thereinto, of the 15 HIV-positive patients, 11 (77.3%) cases had 7 or more features. According to the number of pathological features, another group of specimens with ≤ 6 features was listed. As noted in Table 2, plasma cells, endothelial swelling, epidermis hyperplasia especially irregular acanthosis, and lymphocytes infiltration were the most common findings overall (Figures 1 A–E), although all studied characteristics (Figures 1) had different proportions. Interstitial patterns of inflammation (Figure 1 F) and dermal vasodilatation (Figure 1 B) were relatively common in secondary syphilis. Inflammatory granuloma (Figure 1 K) was relatively rare.
Table 1
Number of histopathological features present for diagnosis. Total specimens = 129
| Features | Case, n (%) |
|---|---|
| 1 | 1 (0.8) |
| 2 | 2 (1.6) |
| 3 | 3 (2.3) |
| 4 | 7 (5.4) |
| 5 | 18 (13.9) |
| 6 | 19 (14.7) |
| 7 | 22 (17.0) |
| 8 | 25 (19.4) |
| 9 | 17 (13.2) |
| 10 | 7 (5.4) |
| 11 | 5 (3.9) |
| 12 | 2 (1.6) |
| 13 | 1 (0.8) |
Table 2
Frequency of histopathological features
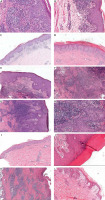
Figure 1
Histopathological features of secondary syphilis. A – Parakeratosis; neutrophils in epidermis; numerous plasma cells with vacuolar pattern (H&E, magnification ×200). B – Endothelial swelling (H&E, magnification ×200). C – Psoriasiform acanthosis (H&E, magnification ×60). D – Irregular acanthosis (H&E, magnification ×60). E – Pseudoepitheliomatous hyperplasia (H&E, magnification ×50). F – Interstitial patterns of inflammation (H&E, magnification ×50). G – Elongated rete ridges (H&E, magnification ×70). H – Neutrophils in epidermis; vacuolar pattern; lymphocytes; plasma cells (H&E, magnification ×100). I – Lichenoid pattern (H&E, magnification ×40). J – Keratinocyte apoptosis (H&E, magnification ×100). K – Inflammatory granuloma in the dermis (H&E, magnification ×30). L – Dermal papilla oedema (H&E, magnification ×100)
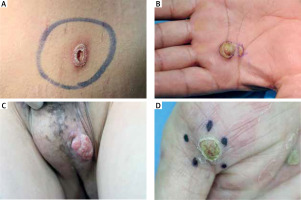
In cases with 6 or fewer features, plasma cells, endothelial swelling, interstitial patterns of inflammation, epidermis hyperplasia were the most common findings, as noted in Table 2. Notably, interstitial patterns of inflammation occurred more frequently in cases with ≤ 6 features than in all cases. The rash morphologies were mainly manifested as macules, maculopapules, plaques and papules with or without scales. Especially in cases with ≤ 6 features, macular and maculopapular rashes accounted for a large proportion, as noted in Table 3. While other rashes demonstrated atypical morphologies including ulcer, nodule, cyst and pustular (Figures 2 A–D), which might be misdiagnosed clinically if not combined with the pathological characteristics of plasma cells (frequency: 100%) and endothelial cell swelling (frequency: 100%) (Table 2). In addition, the treponema pallidum immunohistochemical stain (Figures 3 A, B) was performed on 27 specimens, 20 of which were positive.
Table 3
Demographic features
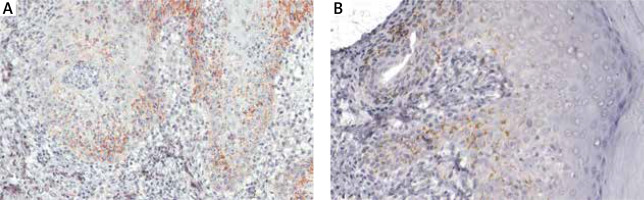
Figure 3
Immunohistochemical stain: T. pallidum was detected at the dermal-epidermal junction, in lower layers of epidermis with an intercellular distribution (A – magnification ×200), partially accompanied by perivascular distribution (B – magnification ×200)
The clinical features and preliminary diagnosis before biopsy are listed in Tables 3 and 4 respectively.
Table 4
Preliminary diagnosis before biopsy
Discussion
Studies have found that the prevalence of syphilis was increasing from 2012 to 2016 [13–15], and there is a continuous increasing trend. Importantly, the proportions of HIV co-infection have been reported to be as high as 25% [16]. In the era of the HIV epidemic, syphilis infection has become a contributing factor to increase the risk of HIV transmission by up to nine fold [4]. This amazing recovery underscores the need for clinicians to be familiar with the myriad of clinical manifestations of syphilis, especially those in its secondary phase. Approximately 25% of untreated syphilis individuals will progress to the secondary form [17], of which about 30% of cases may exhibit atypical eruptions, particularly with HIV infection due to immune dysregulation [18]. The rash of secondary syphilis can be widespread or partial, manifesting as macules, plaques, pustules, papules or scaly; and might mimic other skin diseases including pityriasis rosea, psoriasis, and drug eruptions, etc. Without treatment the lesions of secondary syphilis can disappear spontaneously, taking weeks or months. Moreover, secondary syphilis might progress to the late stage, which can lead to irreversible neurological or cardiovascular complications, blindness and death. The sustained culture of T. pallidum is difficult and usually used only in research. In addition, syphilis serological testing may not be reliable on account of the prozone phenomenon [19, 20]. Nowadays, secondary syphilis still remains a major challenge and a complex diagnosis for the dermatologist and the dermatopathologist alike. Seeking more diagnostic clues for secondary syphilis is necessary. While secondary syphilis is easily misdiagnosed, histopathological features can distinguish secondary syphilis from its mimickers. Previous studies have confirmed that the histopathologic manifestation of secondary syphilis was just as varied as the clinical manifestation [21, 22].
Our patients’ histology revealed that at least one feature or at most 13 features exist in a single biopsy specimen. Most of our cases demonstrated between 5 and 9 diagnostic features. Plasma cells infiltration in the dermis was the most common presentation in 97.6% of all specimens varying from less to numerous in number. And we found it generally tended to have superficial and deep perivascular distribution with or without the lymphocytes, but showing nodular infiltration to form inflammatory granuloma in the minority of cases. The proportion of plasma cells infiltration is apparently higher than in the other two recent studies, by Xiao Ke Liu (86.4%) [23] and by Flamm et al. (70%) [24] separately. The same is true in cases with 6 or fewer features. We still think that this particular feature plays a crucial role for differentiating secondary syphilis from other clinicopathological mimickers, perhaps due to robust cellular and humoral immune responses present in an individual with secondary syphilis.
In addition to plasma cells, endothelial swelling often with dermal vasodilatation, epidermis hyperplasia especially irregular acanthosis, lymphocytes infiltration and interstitial patterns also were the most common findings in all cases as well as in cases with ≤ 6 features. Although endothelial swelling not completely sensitive or specific for syphilis, is present commonly enough in spirochete associated dermatoses to help the pathologist in identifying an atypical infiltrate associated with syphilis in the setting of increased numbers of plasma cells. However, the molecular mechanisms supporting endothelium damage of syphilis have remained undefined. Notably, interstitial patterns of inflammation occurred more frequently in cases with ≤ 6 features than in all cases (72.0% vs. 69.0%), in contrast to the study by Liu et al. [23] (12% vs. 13.6%). The frequencies of keratinocyte apoptosis that is most observed in primary stage lesions and interface dermatitis that is observed more frequently in all cases than in cases with ≤ 6 features were inferior to the other two recent studies mentioned above. Showing once again that plasma cells play the most dominant role, but when there is a lack of the typical clinicopathological feature, the combination of these features can increase the diagnostic clues of secondary syphilis. Granulomatous inflammation is an uncommon histopathologic pattern in secondary syphilis (12.4%), and may be seen in cases as an evolutionary histopathologic change in older lesions of secondary syphilis of varied initial clinical presentation.
Various lesions can exist in 1 patient simultaneously. The rash morphologies of our biopsies mainly manifesting as macules and maculopapules were more likely to have 6 or fewer features, which were not only easily misdiagnosed for pityriasis rosea, tinea and erythema multiforme, but also mostly taken from the trunk and genitalia. Lesions characterized by papules and plaques with or without scales are easily misdiagnosed as psoriasis or condyloma acuminatum clinically, but can be distinguished thanks to significant pathological characteristics. While other rashes demonstrated atypical morphologies including ulcer, nodule, cyst and pustular, which might be misdiagnosed clinically if not combined with the pathological characteristics of plasma cells and endothelial cell swelling. And if feasible, T. pallidum immunohistochemical stain can be done to further confirm the diagnosis. The presence of T. pallidum was detected mostly at the dermal-epidermal junction, mostly in lower layers of epidermis with an intercellular distribution, partially accompanied by perivascular distribution.
When conducting clinical parameter analysis, we concluded that there was no obvious dissimilitude about age and gender distribution as well as diagnosis of syphilis between cases with ≤ 6 features and all cases. In addition, males who tended to be more sexually active still were the majority.
Early accurate diagnosis and timely therapy of secondary syphilis is vitally important for avoiding the morbidity associated with advanced disease. On the basis of seeking more clinical and pathological clues for the diagnosis of secondary syphilis, we can apply these clues to artificial intelligence. Dermatology is a discipline that relies on morphological features. With the continuous development of image technology and digital technology, artificial intelligence is more used in the collection and analysis of digital skin images, discovering internal laws from massive skin image data, assisting clinicians in providing diagnosis and treatment plans for patients, and improving diagnosis accuracy and efficiency. The use of artificial intelligence to assist in the diagnosis of secondary syphilis has long precedents [25, 26]. Therefore, it may be a new method to assist dermatologists in the diagnosis of secondary syphilis by using artificial intelligence to identify histological pictures [27, 28]. Some case report studies also confirmed our results that the pathological features of syphilis can help us make better diagnosis. In study by Kopelman et al. [29] the researchers showed that the skin lesions and dermatopathological analysis showed a pemphigus-like presentation of secondary syphilis. However, other related methods can help pathological studies to make a powerful decision in these patients, for example Müller et al. [30] reported that the combination of PCR and focus-floating microscopy, as the most sensitive method, could provide additional results for the histopathological diagnosis of secondary syphilis and could be useful in cases where serological tests for Treponema pallidum vaccine were ineffective.
Conclusions
The above findings led dermatologists to remain vigilant, consider “the great imitator” in the differential diagnosis, and maintain a high index of suspicion when appropriate, even facing atypical cutaneous presentations. We are convinced that our research data will be able to provide significant reference value for the dermatologist and the dermatopathologist alike.