Introduction
The high mortality and morbidity rates of decompensated cirrhosis have put the treatment and prognosis of this disease in top priority [1, 2]. Establishing the most effective method of treatment based on the patient’s prognosis has been a luxury that hepatologists were not able to afford. A large number of studies have been conducted to elucidate the effects of different inflammatory markers on the survival rate of these patients including the ratio of neutrophils to monocytes, and the ratio of neutrophils to platelets, as well as the distribution of red cell width and its ratio to other blood cells [3]. However, in the past few years, the lymphocyte-to-monocyte ratio (LMR) has been the most studied inflammatory marker. Previous studies have shown the prognostic value of this marker for different diseases, such as cancer, cardiovascular, and gastrointestinal diseases [4-7]. LMR has also been demonstrated to be a good prognostic marker for hepatocellular carcinoma [8]. This marker is being extensively studied due to its cost-effectiveness and ease of calculation and interpretation. Although Pediatric End-stage Liver Disease (PELD), Model for End-stage Liver Disease (MELD), and Child-Pugh scores are commonly used to anticipate the survival chance of children with liver cirrhosis, PELD/MELD scores are difficult to calculate. As an example, to calculate the Child-Pugh score, five parameters including different laboratory data and physical examination are required. This fact has made us look for markers that are easy to obtain, calculate, and interpret. LMR has been widely used for predicting the survival of patients suffering from different chronic diseases, but its role in patients with liver cirrhosis is yet to be studied, and to date, no study has assessed its role in anticipating the prognosis in children with cirrhosis. We conducted this study due to the increasing burden of liver cirrhosis in children and the accessibility of LMR. The primary aim of this study was to focus on the role of LMR in predicting the outcome in children with cirrhosis during their course of hospitalization. Additional aims included studying the relationship of LMR with PELD/MELD and Child-Pugh scores, comparing these three variables in determining patient outcomes, and finding other independent predictors for outcomes in such patients.
Material and methods
All children less than 18 years old who were admitted to the Pediatric Gastroenterohepatology Ward of Nemazee Teaching Hospital (affiliated to Shiraz University of Medical Sciences) due to liver cirrhosis were studied in this project. The diagnosis of liver cirrhosis was based on these criteria: patients who had impaired liver function tests for more than 3 months with any one of the following features: ultrasound findings suggestive of chronic liver parenchymal disease; evidence of decompensated liver cirrhosis with ascites, hepatic encephalopathy, or coagulopathy; previous admissions due to these complications and availability of relevant medical records; or liver biopsy findings suggestive of severe hepatic fibrosis.
The exclusion criteria were as follows: patients aged > 18 years; patients with hepatocellular carcinoma; patients with any other concurrent ailment that could alter LMR, such as the presence of hematological diseases or chronic infections; and patients who had consumed antibiotics in the last 14 days prior to admission.
At the time of admission, informed consent was obtained from patients’ guardians. Blood samples were collected for complete blood count (CBC), prothrombin time (PT)/international normalized ratio (INR), serum albumin, creatinine, serum electrolytes, and liver function tests.
Lymphocyte and monocyte counts were obtained from CBC results, and LMR was calculated by dividing the lymphocyte count by the monocyte count. PELD/MELD scores were calculated using a standard formula available online, and the Child-Pugh score was calculated using five variables of this scale (presence of hepatic encephalopathy, ascites, INR, total bilirubin, and albumin). At the time of admission, LMR, Child-Pugh, and PELD/MELD scores were calculated for each patient. The MELD score was used for patients who were 12 years and older.
The Child-Pugh score includes three continuous numeric variables (total bilirubin, albumin, and PT) and two qualitative variables (encephalopathy and ascites). The variables have been given 1, 2, and 3 points. The overall Child-Pugh score is the sum of these points, ranging from 5 to 15. The score, corresponding to the sum of individual points, allows the categorization of patients in Child-Pugh classes A (5-6 points), B (7-9 points), and C (10-15 points).
The PELD score is calculated using the following formula: PELD score = 0.480 × Loge (bilirubin in mg/dl) + 1.857 × Loge (INR) – 0.687 × Loge (albumin in g/dl) + 0.436, if the patient is less than 1 year old (scores for patients listed for liver transplantation before the patient’s first birthday continue to include the value assigned for age (< 1 year) until the patient reaches the age of 24 months), and 0.480 × Loge (bilirubin in mg/dl) + 1.857 × Loge (INR) – 0.687 × Loge (albumin in g/dl) + 0.667, if the patient has growth failure (less than 2 standard deviations below the mean). Then the calculated number is multiplied by 10 and rounded to the nearest whole number. Laboratory values less than 1.0 are set to 1.0 for the PELD score calculation.
The MELD score is calculated using the following formula: MELD score = 0.957 × Loge (creatinine in mg/dl) + 0.378 × Loge (bilirubin in mg/dl) + 1.120 × Loge (INR) + 0.6431. Then the number is multiplied by 10 and rounded to the nearest whole number. Laboratory values less than 1.0 are set to 1.0 for MELD score calculation.
The patients were followed up during their hospital stay. The patients who were successfully managed and discharged from the hospital regardless of their hospitalization duration were included in the survivor group, and those who died during their stay due to complications of liver cirrhosis were included in the non-survivor group. LMR, PELD/MELD, and Child-Pugh scores were compared between the survivor and non-survivor groups. Additionally, the relationship between these variables was assessed.
The data were analyzed using SPSS version 20 (IBM Corp.; Armonk, NY, USA). Quantitative parameters were expressed in terms of range, mean, and standard deviation, whereas qualitative parameters were expressed in terms of percentages. The p-values for continuous parameters were calculated using sample t-tests, whereas the p-values for categorical parameters were calculated using the contingency coefficient. The receiver operating characteristic (ROC) curve was used to evaluate the efficacy of the three variables (LMR, PELD/MELD, and Child-Pugh scores) in determining the outcome in children with cirrhosis during their hospital stay. The Youden index was used to obtain the cutoff value of each variable, including its sensitivity, specificity, positive likelihood ratio (LR+), and negative LR (LR–). The Pearson correlation was also utilized for these three variables. In addition, the statistical relationship of these three variables for the survivor and non-survivor groups was studied using the χ2 test and Pearson correlation. Binary logistic regression analysis was performed to express the predictors of outcome in these patients.
Results
During 12 months, 132 cirrhotic children were admitted to the Pediatric Gastroenterohepatology Ward. Of these, 18 were excluded: 3 had hepatocellular carcinoma and the other 15 cases had taken antibiotics at the time of admission. 114 patients consisting of 55 (48.2%) boys and 59 (51.8%) girls were included in the study. The mean ±SD age of patients was 68.1 ±63.1 months (range: 1.5-204) and the mean ±SD weight was 20.6 ±17.7 kilogram (range: 3.2-85). The most common causes of cirrhosis were biliary atresia (n = 38, 33.3%) and Wilson disease (n = 27, 23.7%). Other causes were autoimmune hepatitis (n = 10, 8.8%), tyrosinemia (n = 9, 7.9%), other metabolic diseases (n = 8, 7%), progressive familial intrahepatic cholestasis (PFIC) (n = 8, 7%), neonatal hepatitis (n = 4, 3.5%) and miscellaneous (n = 10, 8.8%).
The most common complications were ascites (n = 80, 70.2%) and variceal bleeding (n = 36, 31.6%), followed by encephalopathy (n = 31, 27.2%), pruritus (n = 10, 8.8%), hepatorenal (n = 2, 1.8%) and hepatopulmonary syndromes (n = 1, 0.9%). During hospital stay 26 patients (22.8%) died. The baseline characteristics of the study group regarding outcome are shown in Table 1.
Table 1
Baseline characteristics of 103 patients in the study group regarding outcome
Out of 114 patients 8.8% were in Child-Pugh class A, 29.8% in class B and 57.9% in class C. The mean ±SD Child-Pugh, PELD/MELD scores and LMR were 9.7 ±2.3 (1-14), 22.8 ±11.5 (1-58) and 14.5 ±14.6 (1.4-86), respectively. Child-Pugh and PELD/MELD scores were positively correlated (r = 0.481, p < 0.001), whereas LMR had a strong negative correlation with PELD/MELD (r = –0.87, p = 0.36) and a weak negative correlation with Child-Pugh scores (r = –0.046, p = 0.63).
The highest area under the curve (AUC) belonged LMR [AUC = 0.861 with 95% CI ranging from 0.772 to 0.950 (p < 0.0001)]. The AUC was also good for PELD/MELD score [AUC = 0.804, 95% CI = 0.694-0.914, p < 0.0001]. The AUC for Child-Pugh score was 0.655 with 95% CI ranging from 0.537 to 0.773 (p = 0.017). Figure 1 shows the ROC curve of the three variables (LMR, PELD/MELD and Child-Pugh scores). The AUC was not statistically significantly different between LMR and PELD/MELD scores, suggesting that both variables can be used to assess the outcomes in patients with liver cirrhosis during their hospital stay.
Fig. 1
ROC curve for LMR, Child-Pugh and PELD/MELD scores for predicting outcome
Diagonal segments are produced by ties.
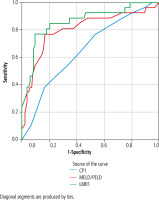
Table 2 shows the mean LMR, Child-Pugh, and PELD/MELD scores in association with outcome in survived and non-survived groups. The differences between LMR and the other two methods were significant.
Table 2
Mean LMR, Child-Pugh and PELD/MELD scores in association with the outcome
Because the white blood cells (WBC) differential of children at 6 years of age becomes the same as adults, we divided the patients according to age into two groups: younger than 6 years and older than 6 years. 67 patients (58.8%) were younger than 6 years and 47 patients (41.2%) were older. Table 3 shows the mean LMR in association with the outcome in these two age groups. The AUC for LMR in the younger group was 0.675 (95% CI: 0.462-0.888) (p = 0.111) and in patients older than 6 years it was 0.926 with 95% CI ranging from 0.852 to 1.000 (p < 0.001).
Table 3
Mean LMR in association with outcome in two age groups
| Age group | Outcome | N | Mean | SD | P value | 95% CI | |
|---|---|---|---|---|---|---|---|
| ≤ 6 yr. | LMR | Survive | 59 | 18.44 | 17.47 | 0.181 | –4.062-21.120 |
| Death | 8 | 9.91 | 8.50 | ||||
| > 6 yr. | LMR | Survive | 29 | 14.95 | 9.22 | 0.000 | 8.213-15.355 |
| Death | 18 | 3.16 | 1.53 |
The cutoff value for LMR was calculated using the Youden index, which that was 4.6 [Youden index = 66.7%, sensitivity = 76.9% (95% CI = 56.4-91.0), specificity = 89.8% (95% CI = 81.5-95.2), LR+ = 7.52 (95% CI = 3.9-14.5), and LR– = 0.26 (95% CI = 0.1-0.5)]. Considering these values, the patients were divided into two groups: low (≤ 4.6) and high (> 4.6) LMR. A total of 29 patients (25.4%) had low LMR, whereas 85 (74.6%) had high LMR (Table 4).
Table 4
Relationship between PELD/MELD and Child-Pugh scores with LMR
| Scores | LMR | N | Mean | SD | P value | 95% CI |
|---|---|---|---|---|---|---|
| PELD/MELD | Low | 29 | 29.04 | 12.97 | 0.001 | 3.74-13.04 |
| High | 85 | 20.66 | 10.14 | |||
| Child-Pugh | Low | 28 | 10.04 | 1.87 | 0.376 | –0.554-1.455 |
| High | 82 | 9.58 | 2.44 |
The cutoff value for PELD/MELD score was > 26 [Youden index = 59.9%, sensitivity = 76.9%, (95% CI = 56.4-91.0), specificity = 82.9% (95% CI = 73.4-90.1), LR+ = 4.51 (95% CI = 2.7-7.5), LR– = 0.28 (95% CI = 0.1-0.6)]. The cutoff value for Child-Pugh score was > 9 [Youden index = 23.3%, sensitivity = 76.9% (95% CI = 56.4-91.0), specificity = 46.4% (95% CI = 35.5-57.6), LR+ = 1.44 (95% CI = 1.1-1.9), LR– = 0.50 (95% CI = 0.2-1.0)].
The PELD/MELD scores were significantly higher in the low LMR group than in the high LMR group (p = 0.001). It was also found that Child-Pugh score was higher in the low LMR group but its difference was not statistically significant (p = 0.148) (Table 4). Regarding Child-Pugh class; 10%, 29.4%, and 37.9% of patients in classes A, B, C had low LMR, respectively. As a result, many patients with Child-Pugh class C had low LMR. Similarly, the low LMR group had lower survival compared with the high LMR group (33.3% vs. 92.9%) (p = 0.000).
Both logistic regression and correlation analyses were used to define the relationship between the scores and outcomes in patients during a hospital stay. Positive correlations were found between the three variables and patient outcomes [Child-Pugh score (r = 0.221, p = 0.020), PELD/MELD scores (r = 0.449, p = 0.000), and LMR (r = 0.571, p = 0.000)]. All the parameters were tested using logistic regression to find possible predictors for outcomes in patients during hospital stay. The positive predictors for outcome in these patients were PELD/MELD score (OR = 1.140, 95% CI = 1.063-1.222, p = 0.000) and LMR (OR = 0.831, 95% CI = 0.748-0.923, p = 0.001).
Discussion
The present study is, to our knowledge, the first one regarding the role of LMR in predicting the outcome in children with cirrhosis during their hospital stay. Utilizing the proper prognostic scale in a disease, and choosing the treatment based on the prognosis acquired by the scale can, in turn, enhance the overall prognosis of the disease. This fact can be a matter of life and death in patients in need of transplantation such as those living with cirrhosis [9]. Multiple studies have been conducted to determine the best prognostic scale in pediatric cirrhosis [10]. Although PELD/MELD and Child-Pugh scores have been widely used to serve this purpose, each scoring system has certain limitations [11]. CBC is an inexpensive and easy-to-perform diagnostic test, widely used in everyday clinical practice. It is very important in diagnosing and monitoring different diseases. Recently, several studies focused on the proportion of different types of leukocytes in various medical conditions [12]. The utilization of LMR as a prognostic marker is under close investigation in gastrointestinal cancers [13]. Also, there is strong evidence for its significance as a prognostic factor in cardiovascular diseases and solid tumors [14]. Systemic inflammatory response syndrome (SIRS) with no apparent source of infection is the key element in the pathogenesis of circulatory dysfunction due to advanced cirrhosis. Consequently, this phenomenon is shown to be of great importance in determining the outcomes of cirrhotic patients [15]. SIRS is a major factor in worsening the conditions of stable cirrhotic patients, thus exposing them to the risk of different complications, and even early death [16]. Monocytes are the most important cells of the immune system that can cause liver fibrosis. Endotoxins can trigger the activation of monocytes and the release of various cytokines which further recruit other blood cells. This cycle repeats over and over again and can result in circulatory alterations and liver fibrosis [17]. The existence of monocytosis and its positive association with disease progression have been proved previously in cirrhotic patients [18]. Moreover, lymphopenia has been documented in many diseases such as malignancy, tuberculosis, viral infection, and peripheral vascular disease [19, 20]. Liver disease can also predispose patients to lymphopenia. Thus, patients with liver disease can experience an imbalance in their immune system due to alterations in monocyte and lymphocyte counts [21]. There is a close relationship between cirrhosis and immune dysfunction. The immune system either in its innate or adaptive component is dysregulated in cirrhosis. This is called a state of acquired immunodeficiency. In cirrhosis there is a disruption of the immune function at both levels: locally in the liver itself and systematically [6]. The microbicidal capacity of neutrophils and chemotaxis is impaired. The function, distribution, and number of monocytes are also altered in cirrhosis. According to the severity of liver disease, the monocyte pool will increase but with impaired phagocytic capacity. Lymphocytes are also affected by cirrhosis. Memory cell dysfunction is the most striking abnormality [6]. Numerous studies have been conducted to assess the role of inflammatory cells and markers in determining the prognosis of chronic diseases including liver cirrhosis. They showed that both monocyte and lymphocyte counts are disrupted. The lymphocyte count will decrease, but on the other hand, monocytosis is positively correlated with the disease progression [22].
To determine the prognosis in cirrhotic patients, several markers have been used including the ratio of neutrophils to monocytes, neutrophils to platelets, and distribution of red blood cell width and its ratio with other blood cells. One of the studied inflammatory markers that is cost-effective and easy to calculate is the LMR. This marker is not only useful in cirrhotic patients but it also has a significant role in determining the survival of patients with various diseases, such as cancer, cardiovascular disease, gastrointestinal diseases (Crohn disease), and colorectal carcinoma [22]. In the present study, the mean Child-Pugh, PELD/MELD scores and LMR were 9.7, 22.8, and 14.5, respectively. Child-Pugh and PELD/MELD scores were positively correlated (r = 0.481, p < 0.001). With the disease progression, these scores will be increased, whereas LMR had a strongly negative correlation with PELD/MELD scores (r = –0.87, p = 0.36) and a weakly negative correlation with Child-Pugh score (r = –0.046, p = 0.63). These findings show that the lower the LMR is, the higher the PELD/MELD score would be. Our findings were in line with those of Jamil et al. [22] and Zheng et al. [23]. In addition, by using ROC analysis, the predictive value of these three scores in assessing the survival of cirrhotic patients was evaluated. It showed that LMR has the highest AUC compared to PELD/MELD and Child-Pugh scores (LMR = 0.861, PELD/MELD = 0.804, Child-Pugh = 0.655). This is compatible with what was found in other studies [19, 20]. The AUCs of both LMR and PELD/MELD scores were similar, both being statistically significant. This means that both LMR and PELD/MELD scores can be used equally in determining the prognosis of pediatric patients with cirrhosis, whereas the Child-Pugh score has a less significant value as a prognostic marker in these patients.
LMR score also has high clinical utility in predicting bacterial occurrence in patients with liver cirrhosis according to Piotrowski et al. [24]. The roles of LMR, PELD/MELD, and Child-Pugh scores were compared in the surviving and the non-surviving group, as well, which showed that, using each of these criteria the difference between the two groups was statistically significant. It is important to mention that PELD/MELD and Child-Pugh scores can be calculated quickly and not only assess liver function but also yield predictive information. However, they represent only one point in time and do not take into account the full clinical picture [25].
In the pediatric age group under normal circumstances, lymphocytosis is more common. With increasing age lymphocytes account for 35%, and granulocytes account for 65%. Next, lymphocytes can reach 50%, which is roughly equal to the number of granulocytes. In 4- to 6-year-old children the proportion of lymphocytes decreases, and the proportion of granulocytes increases, close to the adult level [26]. In the present study 67 patients (58.8%) were less than 6 years and 47 patients (41.2%) were older. The AUC for LMR in patients less than 6 years old was 0.675 with 95% CI ranging from 0.462 to 0.888 (p = 0.111), and in older patients it was 0.926, with 95% CI ranging from 0.852 to 1.000 (p < 0.001). In other words, LMR is a good prognostic marker for in-hospital mortality in cirrhotic children older than 6, but its utility for patients aged less than 6 years is questionable.
To date, no study has assessed its role in anticipating the prognosis in children with cirrhosis, and this is the main strength of the current study. The main limitation was the sample size and being a single-center study.
Conclusions
Lymphocyte-to-monocyte ratio can be used to determine the outcome of cirrhotic children older than 6 years during the hospital stay because it is easy to calculate, cost-effective, non-invasive and its efficacy is comparable to PELD/MELD scores. Meanwhile, further studies are needed to confirm these preliminary results.






