Introduction
According to the TIME strategy, the first step of the local treatment of the wound is debridement. A number of different methods are available from the use of a bone spoon to advanced ultrasound devices [1–5]. Some of these methods are impossible to be used in outpatient settings, not to mention usage by the patient or their caregivers. Modern wound cleaning products such as sterile cloths or sponges are available on the market. It is not known, however, whether these products have an advantage over the use of sterile gauze as the testing of individual products is sponsored [6]. That is why the aim of this study was to test 3 different products for debridement, which could be easily used by the patients or their caregivers themselves, on a selected group of patients with solely venous ulcers.
One of the products subjected to this research is a non-woven cloth impregnated with a solution of sodium hyaluronate, aloe vera and phospholipids (CleanWnd®). It contains short fibres enabling it to collect wound debris easily during mechanical debridement without leaving any residual lint. CleanWnd® has been designed to cleanse and debride wounds with a solution of Sodium Hyaluronate and Phospholipid which is suitable for wound cleansing at every dressing change. CleanWnd® non-woven impregnated cloth is manufactured by the needle punch method and contains short fibres enabling it to collect wound debris easily during mechanical debridement without leaving any residual lint. Hyaluronic acid is a vital compound found in extracellular matrix, which triggers wound healing and tissue repair process [7–10]. On the other hand, phospholipids act as a surfactant that eases cleansing and replaces some of the phospholipids lost by damaged tissue [11, 12].
The second debridement tool tested is a sterile sponge (blue Schülke Wound Pad®) that is designed to be soaked in a surfactant solution (Octenilin®). Schülke Wound Pad wound dressings are made of flexible, foamed polyurethane with a coarse-grained structure and a rough surface. As those wound dressings differ by the number of pores and roughness, the medium one was chosen to be compared with a cloth. It is characterised by medium pores and medium fluid permeability coefficient, which enables removal of fibrin and excessive exudate from the wound surface, for example, hyperkeratotic skin or residual dressings [13]. Sponges improve blood circulation and oxygen supply to tissues, which increases the strength of granulation and spontaneous cleansing of the wound, without the intervention of a surgeon [14].
The third debridement method is monofilament fibre cloth (Debrisoft Pad®). Cloth is knitted by millions of angled, soft, polyester fibres attached to polyacrylate backing and sewn edges.
It is suitable for allergic patients as it contains no chemically irritant substances. Debrisoft® is suitable for a rapid and atraumatic debridement with first visible results in 2 to 4 min [15].
Fibre composite lifts, binds and therefore eases to remove slough, including biofilm and non-viable debris [16, 17]. Due to bevelled fibre tips, cloths effectively cleanse, while protecting new granulation tissue and epithelial cells [18, 19]. According to the manufacturer, Debrisoft Pad® should be hydrated with a saline solution before use.
Material and methods
The study group consisted of 32 patients randomly assigned to 4 different groups (n = 8), according to the debridement method: sterile sponge (blue Schülke Wound Pad®), monofilament cloth (Debrisoft Pad®), non-woven impregnated cloth (CleanWnd®) and sterile gauze debridement procedure. Data collected during the interview and treatment results collected during follow-up examinations were entered into the database. Each patient was given a number. Randomization of patients and assignment to a group was determined in order of application. Patients’ data were coded in accordance with the Personal Data Protection Act. Approval of the Independent Bioethics Commission for Research at the Medical University of Gdansk to conduct this research has been received (Bioethics Commission Agreement no. NKBBN/601/2019).
After reading written information about the purpose and method of conducting the study, obtaining comprehensive answers to the questions asked and after signing the written consent to participate in the study, the patient was qualified for the study.
The primary end point of the study was the level of sloughy tissue removal, whereas the secondary end point was the degree of granulation of the wound. The study ended when the wound was completely debrided of necrotic tissue or after 30 days. In addition, this study assessed the impact of the applied treatment on ease of local wound care, development of ulcer infection, presence of hypersensitivity reactions, pain during surgery (VAS), cleaning time, cost-effectiveness and the rate of cleaning the wound from necrotic tissue.
During the inclusion visit, a thorough medical history was taken and a physical examination was performed as per the schedule. The inclusion and exclusion criteria were to be established on the basis of the anamnesis and physical examination (Table 1). Assessment of the ulcer size was carried out using a specially prepared 0.5 cm film and photographic documentation was taken at intervals of 7 days. One photograph was taken before and the other after the wound cleansing procedure. The surface area of the ulcer as a whole, exudates + necrosis and granulation tissue was measured using planimetry tools.
Table 1
Inclusion and exclusion criteria
Shin hygiene was carried out with the help of washing and conditioning foam cleaning, then depending on the group, the debridement procedure was carried out with the use of Schülke Wound Pad® soaked in Octenilin® liquid, monofilament cloth Debrisoft Pad® soaked in saline solution, non-woven impregnated with sodium hyaluronate and phospholipid cloth CleanWnd® or sterile gauze. Consequently, decontamination of the wound with hypochlorite preceded the application of the dressing – hydrogel with hydrocolloid and alginate (Purilon®, Coloplast) as a primary and non-adhesive polyurethane foam dressing (Biatain non-adhesive®, Coloplast) as a secondary dressing. The use of combination of those two dressings is advisable, for hydrocolloids with alginates aid necrosis removal, while foam dressings prevent dressing-related trauma and absorbs excessive wounds exudate, providing optimal wound healing conditions [20–25]. Finally compression therapy with short stretch bandages was applied.
Patients received dressings, the debridement product tested and compression bandages. Furthermore, they were instructed how to use the products received and the first dressing was to be applied by the doctor participating in the study. Dressings were to be changed twice a week, once by the patient and the other time by the physician.
The patient was notified of the need to report for follow-up appointments in accordance with the protocol (every 7 ±1 day), or contact the doctor conducting the examination in case of adverse effects (pain, swelling, redness of the skin around the ulcer, increase in body temperature).
Statistical analysis
Baseline patient characteristics and clinical outcomes will be compared between different debridement methods tested: sterile gauze, sterile sponge, monofilament cloth and non-woven impregnated cloth.
Photographs were taken with a phone camera with a high resolution (iPhone 11Pro, Apple, California, USA). Statistical analyses were performed using the SPSS for MacBook package program (version 26, IBM Corp. Armonk, New York, USA). Groups were tested for statistical significance of the values representing relative reduction of total wound area between day 0 to day 30, necrotic tissue area reduction after first debridement procedure and between day 0 before the debridement and day 30 after the debridement.
In all analyses, p < 0.05 was used to indicate statistical significance.
Due to compliance with the normal distribution of most variables measured with Shapiro-Wilk test, Anova analysis and post-hoc Bonferroni test were used to determine comparisons between the groups. Furthermore, the relationship between the variability of the effectiveness of the new non-invasive mechanical cleansing methods at different time periods was examined with the assessment of patient satisfaction, cleaning time and pain level measured with the VAS scale. The correlations between the groups were calculated using the Pearson correlation coefficient test.
Results
All 32 patients enrolled into the study completed it. Characteristics of the study group at the admission have been shown in Table 2.
Table 2
Baseline characteristics and medical history of the study groups
| Parameter | Sterile gauze | Blue Schülke Wound Pad® | Debrisoft Pad® | CleanWnd® |
|---|---|---|---|---|
| Male (n) | 4 | 4 | 2 | 5 |
| Female (n) | 4 | 4 | 6 | 3 |
| Age (mean ± SD) [years] | 73.87 ±10.81 | 74.25 ±13.53 | 80.37 ±9.39 | 75.27 ±15.30 |
| Previously healed ulcer (n) | 4 | 2 | 4 | 1 |
| Phlebectomy (n) | 0 | 0 | 2 | 1 |
| Wound duration [months] | 14.63 | 20.50 | 25.50 | 22.50 |
| Wound duration (median) [months] | 11.50 | 13.50 | 24.00 | 16.50 |
| Wound area (mean ± SD) [cm2] | 40.43 ±30.08 | 38.20 ±25.28 | 43.04 ±74.53 | 54.86 ±63.88 |
| Wound area (median) [cm2] | 34.13 | 31.68 | 14.36 | 20.96 |
| P-value (SW) | 0.182 | 0.086 | 0.000 | 0.029 |
| Clinical status of the wound bed tissue: | ||||
| Necrotic tissue (mean value) | 85.52% | 84.58% | 76.42% | 91.00% |
| Granulation tissue (mean value) | 14.48% | 15.42% | 23.58% | 9.00% |
| Mean exudate abundance (scale 0–5) | 4.00 | 4.13 | 4.13 | 3.88 |
| Periwound skin condition (n): | ||||
| Hyperkeratosis | 5 | 5 | 1 | 1 |
| Maceration | 3 | 0 | 5 | 1 |
| Eczema | 1 | 3 | 5 | 3 |
| Signs of inflammation in the wound (n): | ||||
| Redness | 0 | 1 | 1 | 4 |
| Swelling | 2 | 4 | 3 | 3 |
| Odour | 3 | 2 | 2 | 2 |
| Healing cessation | 0 | 0 | 0 | 0 |
| Pain within the wound | 0 | 0 | 2 | 0 |
| Average cost of the debridement method for 30 days [PLN] | 3.78* | 295.20* | 114.30* | 55.44** |
The mean wound area at day 0 (cm2) was 40.43 ±30.08 for gauze, 38.20 ±25.28 for blue Schülke Wound Pad®, 43.04 ±74.53 for Debrisoft Pad®, 54.86 ±63.88 for Clean Wnd® (Table 2). Mean necrotic tissue abundance (% of total wound area) was 85.52 for gauze, 84.58 for blue Schülke Wound Pad®, 76.42 for Debrisoft Pad®, 91.00 for Clean Wnd®.
Wound area distribution in the majority of the study groups had either a distribution consistent with the normal distribution or not statistically different from the normal distribution (p > 0.05). Anova comparative analysis of variance showed that the study groups did not differ significantly in terms of the total wound area at day 0 (p = 0.924). For those reasons the relative reduction in both the total wound area and necrotic tissue between the study groups could have been compared.
In order to compare the efficacy of all the products, the relative reduction in the total wound area between day 0 to day 30 and necrotic tissue area after the first debridement procedure and between day 0 before the debridement and day 30 after the debridement was calculated.
The comparison of the reduction of the wound area on day 30 compared to day 0, shows an average 20.69 ±21.93% reduction in the gauze group, 23.55 ±27.38% for the Schülke Wound Pad® group, 15.41 ±25.72% for the Debrisoft Pad® group and 12.51 ±17.95% for the CleanWnd® group (Table 3).
Table 3
Descriptive statistics, results of Shapiro-Wilk normality analyses and Anova analysis of variance comparisons for study groups with relation to the variability of the wound area and necrotic tissue over time and in terms of the satisfaction level, wound cleaning time and pain level
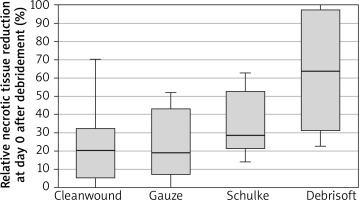
Relative necrotic tissue reduction at day 0 after debridement was 23.53 ±19.16% in the gauze group, 34.11 ±17.51% for the Schülke Wound Pad® group, 63.44 ±32.91% for the Debrisoft Pad® group and 23.56 ±22.15% for the CleanWnd® group (Table 3, Figure 1).
It was shown that there the differences between the groups in terms of the relative reduction in necrotic tissue after a single wound debridement on day 0 were statistically significant (p < 0.01; η2 = 0.35).
A series of comparative analyses with post hoc tests have shown statistically significant differences (p < 0.05) between the reduction of necrotic tissue at day 0 in the group subjected to debridement with Debrisoft Pad® than in the other groups of patients subjected to debridement with other methods (Table 3). On the other hand, no statistically significant differences were found in the reduction in necrotic tissue during a single cleaning between the remaining groups (Schülke Wound Pad® vs. CleanWnd®) and in relation to Schülke Wound Pad® vs. gauze or CleanWnd® vs. gauze.
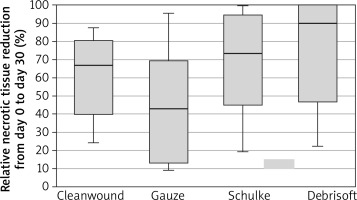
Relative necrotic tissue reduction at day 30 compared to day 0 was 44.95 ±31.47% in the gauze group (Figure 2), 68.38 ±28.78% for the Schülke Wound Pad® group (Figure 3), 74.65 ±30.95% for the Debrisoft Pad® group (Figure 4) and 60.90 ±23.44% for the CleanWnd® group (Table 3, Figures 5, 6).
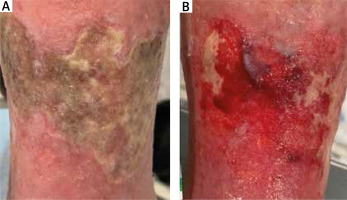
Figure 2
Patient subjected to debridement with sterile gauze: A – before debridement at day 0, B – after debridement at day 30
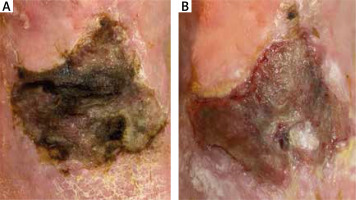
Figure 3
Patient subjected to debridement with Schülke Wound Pad®: A – before debridement at day 0, B – after debridement at day 30
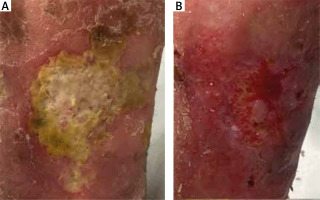
Figure 4
Patient subjected to debridement with Debrisoft Pad®: A – before debridement at day 0, B – after debridement at day 30
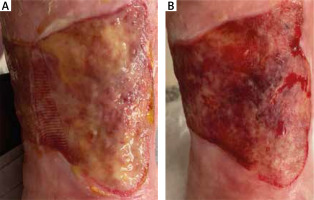
Figure 5
Patient subjected to debridement with CleanWnd®: A – before debridement at day 0, B – after debridement at day 30
According to the Anova test, there was no statistical difference between the groups in the reduction of necrotic tissue over 30 days (p = 0.216; η2 = 0.14), yet a series of comparative analyses with post hoc tests showed that there were statistically significant differences (p < 0.05) between the reduction in necrotic tissue over the period of 30 days in the group between the Debrisoft Pad® group and the group with the gauze. There were no statistically significant differences between the other groups (CleanWnd® vs. gauze, Schülke Wound Pad®), vs. gauze, or Debrisoft Pad® vs. CleanWnd® vs. Schülke Wound Pad®), which suggests that other products tested might not have been more efficient than sterile gauze in necrotic tissue reduction.
Furthermore, the correlation between the variability of the effectiveness of the new non-invasive mechanical cleansing methods at different time periods was examined in relation to the assessment of patient satisfaction, cleaning time and pain level measured using the VAS scale. The correlations were calculated for the general group of test persons using a series of Pearson r correlation analyses.
The analysis of variance performed showed that the satisfaction with treatment in the study groups was statistically significantly different (p < 0.001; η2 = 0.69) (Table 3). A series of comparative analyses using Bonferroni post hoc tests showed that patients in the gauze group reported a lower satisfaction with treatment at 4.75 ±0.89 points (in scale 0–10) than those using other methods of debridement, and the differences were significant (8.13 ±0.83 for the Schülke Wound Pad® group, 7.5 ±0.93 for the Debrisoft Pad® group and 7.5 ±1.07 for the CleanWnd® group).
It was also shown that there were statistically significant differences between the study groups in the time of cleaning the wound at day 0 (p < 0.001; η2 = 0.75) and at day 30 (p < 0.001; η2 = 0.60) (Table 3). In both cases, a series of post hoc post hoc analyses of Bonferroni showed that the cleaning time for the CleanWnd® group was longer than for the other study groups. The time of cleaning the wound in the group of people using CleanWnd® in the first measurement was over 11 min, and in the remaining groups it did not exceed 10 min, on average was 5 min.
It was shown that the study groups differed statistically significantly in terms of the assessment of the pain scale during the procedure at day 30 (p < 0.01; η2 = 0.46). Multiple post hoc Bonferroni comparisons showed that people using gauze and CleanWnd® rated their pain levels higher than those using Schülke Wound Pad®, and Debrisoft Pad®.
A series of Pearson’s r-correlation analyses showed that there was a statistically significant correlation between the level of pain on the VAS scale with the relative reduction of necrotic tissue after debridement at day 0 (p < 0.05) and with the reduction of necrotic tissue from day 0 to 30 (p < 0.05). Correlations were negative which means that people who had a greater relative reduction of the necrotic tissue after cleansing both at day 0 and day 30 in relation to day 0 had less pain intensity on the VAS scale. These correlations were of moderately strong intensity.
Discussion
This study was the first one to compare 3 different non-invasive mechanical debridement methods – sterile sponge, monofilament cloth and non-woven cloth impregnated with sodium hyaluronate and phospholipids in relation to traditional sterile gauze.
The only studies on non-invasive mechanical debridement include small pilot, non-comparative studies, which suggested good results in removing sloughy, necrotic tissue after one use. The aim was to determine if there is any statistically significant difference between 3 products tested and in relation to sterile gauze, which is the first example of the non-invasive mechanical method.
As it comes to limitations of our study, we admit that our study groups were rather small as the study was conducted during COVID-19 pandemic. Undoubtedly, randomised controlled trials on a bigger study sample would be advised and could contribute to confirmation of our initial results.
As the results have shown, monofilament cloth – Debrisoft Pad® might be the most effective among those. This means that Debrisoft Pad® could be potentially seen as an effective successor of sterile gauze, which provides a rapid and easy-to-use debridement method with a lower pain level during cleaning.
Even though that both CleanWnd® and Schülke Wound Pad® have not shown any statistically significant advantage over sterile gauze, when it comes to efficacy in relative necrotic tissue reduction, there were significant differences in the pain level during the procedure and the patient’s satisfaction level. Patients using Schülke Wound Pad®, and Debrisoft Pad® rated their pain levels in VAS lower than patients using sterile gauze. Moreover, patients using all 3 products tested reported higher satisfaction with treatment (0–10 scale) than patients treated with sterile gauze. As ulcer treatment is unpleasant itself, the highest aim should be to raise patient’s satisfaction with the treatment. This is why, using three novel methods tested can be suggested as they are well accepted by patients and cause less pain during the procedure, which is essential for good compliance and complete resolution of the lesions.
A relatively low price of debridement with the use of CleanWnd® is also worth noticing. 30 days’ debridement performed twice a week is consequently approximately PLN 3.78 for sterile gauze, PLN 295.20 for Schülke Wound Pad®, PLN 114.30 for Debrisoft Pad® and PLN 55.44 for CleanWnd®. Even though the price of Debrisoft Pad® is higher than the price of sterile gauze, patient satisfaction and efficacy should be taken first into consideration, when choosing the debridement method.
Results of the study have not shown any statistically significant difference in relative wound area reduction from day 0 to day 30 between the groups studied. Even though this was not the main aim of the research, possibly extending the study duration to at least 3 months, simultaneously adding application of a wound pad that accelerates epithelialization, after the wound has fully granulated, could result in obtaining more statistically significant differences in wound area reduction between different non-invasive mechanical debridement methods used.
The European Wound Management Association included information about Debrisoft Pad® as a mechanical debridement method in its guidelines of 2013. Surely more methods available on the market should be added to both European and international guidelines, which would meet the new TIMERS guidelines of 2019, which replaced previous TIME guidelines [26]. Both acronyms stand for structured guidance on wound management approaches that optimize healing outcomes. The acronym TIME, where T stands for tissue debridement, I for inflammation and infection control, M for moisture balance, E for wound edges and epithelial advancement, was upgraded to TIMERS, where R stands for repair and regeneration and S for social and individual-related factors. The biggest change with TIMERS guidelines was adding the need of good compliance with the patient by adding the letter R [26].
Non-invasive methods are such methods as they enable both the patient or his/her guardian or nurse to use them to achieve a beneficial outcome of the treatment and higher patient satisfaction. This could undoubtedly lower the costs of hospitalization or sick leaves of those patients and contribute to better quality of life.