Introduction
Chronic venous insufficiency (CVI) is a disease of the venous system of the lower extremities, where chronic venous hypertension is a prerequisite for its development, leading to various pathologies, including pain, oedema, skin changes (discoloration, eczema, lipodermatosclerosis, white skin atrophy) and active or healed shin ulcers. Due to the accompanying insufficiency of the venous valves and elevated venous pressure, the term “CVI” is used to describe the full spectrum of symptoms of chronic venous disease (CVD) [1–6]. Chronic venous disease is a serious social problem as it affects a significant percentage of the European population, including 30–60% of women and 15–30% of men in the Caucasian population, with slightly lower rates in people of Spanish, African-American and Asian origin [1–4, 7]. Its frequency clearly increases with the age of the respondents. Based on the research conducted by Jawieñ et al. [8], in the Polish population, in a cohort of 40,095 primary care patients (PCP), signs and symptoms of CVI were reported in 47% of women and 37% of men.
The risk factors for the development of CVI include gender, old age, family history, standing position in work conditions, obesity, smoking, sedentary lifestyle, injuries of the lower limbs, previous venous thrombosis, presence of arteriovenous shunt, high oestrogen levels and pregnancy [7, 9–11].
The main cause of pathology development both within the venous system of the lower limb and in the surrounding tissues is venous hypertension caused by valvular reflux, narrowing and slowing of venous flow, or both, which, with the participation of risk factors, lead to the development of varicose veins and formation of venous leg ulcers, which are the final stage of chronic venous insufficiency [12–15].
Hypertension and stagnation of venous blood in microcirculation are accompanied by the phenomena of adhesion, migration and activation of leukocytes (the so-called leukocyte trap), which damage the microcirculation vessels. Excessive capillary permeability and inflammatory mediators damage the capillary barrier, which leads to limb oedema and impairment of tissue nutrition [16–19].
The variety of haemodynamic symptoms present in CVI and the observed clinical symptoms indicate the presence of inflammation secondary to venous hypertension and leading to the activation of many inflammatory pathways. The endothelium and the glycocalyx, through the presence of specialized receptors, are crucial in detecting changes in vascular wall tension, and the expression of adhesive molecules enables the activation of leukocytes, leading to their adhesion to the endothelium, diapedesis and transmigration to the venous wall/valves, which leads to damage to the venous wall and the presence of cell inflammation in the perivascular tissue. The comprehensive action of cytokines, chemokines, growth factors, proteases and proteinases produced by activated leukocytes that are expressed and disrupt tissue homeostasis, resulting in a persistent inflammatory environment with frequently observed clinical changes, ranging from varicose veins to more advanced skin symptoms and venous ulcers. The structural integrity of the protein and the extracellular matrix is altered, intensifying the progressive pathological changes in CVI [20, 21].
Endothelial dysfunction is now believed to result from early endothelial dysfunction associated with glycocalyx damage and induced by inflammatory cells and mediators (such as matrix metalloproteinases and interleukins) that lead to progressive dilation of the veins resulting in CVI. During the inflammatory process, activated leukocytes release enzymes, free radicals, chemokines and inflammatory cytokines in the vascular microenvironment, which are responsible for changes in the venous wall and venous valves, reflux and venous hypertension, as well as progressive tissue damage and the development of skin lesions [22–24].
The local development of inflammatory processes is usually accompanied by a systemic reaction known as acute phase response. It arises as a result of various damaging stimuli; chemical-, thermal- or mechanical trauma as a result of infections, parasite infestation, physical exertion and high mental stress. In modulating the acute phase reaction, cytokines play the role of cell growth factors and inflammatory mediators with pro-inflammatory and anti-inflammatory effects. They are produced and released by activated cells of the immune system.
Acute phase proteins (APP) have various biological functions: they activate the classical complement pathway (CRP); by activating proteases, they prevent tissue damage (α1-ACT, α1 antitrypsin (AAT), α2-MG); participate in the opsonisation of bacteria and damaged tissue fragments (CRP and SAA); they also participate in the processes of fibrinolysis and coagulation (fibrinogen, AAG); they are also responsible for the regeneration of healing tissues and participate in the reorganization of newly formed collagen fibres (AAG); they also prevent the body from losing iron (Hp, Tf) [25, 26].
IL-1, TNF-α and IL-6 are primarily responsible for initiating the synthesis of APP as a result of a damaging or inflammatory stimulus. CRP is of particular importance among APP as it is the most frequently determined indicator of inflammation. CRP is mainly synthesized by hepatocytes; its expression is also found in lymphocytes and monocytes; it is also exhibited by macrophages and smooth muscle cells within the atherosclerotic plaque [27, 28].
CVD, like CVI, is associated with valve dysfunction, venous hypertension, and endothelial inflammation. New theories on the pathophysiology of CVI recognize inflammation, endothelial glycocalyx dysfunction, and the resulting extracellular matrix alterations as a key in the development of CVI and increase interest in research into the use of Sulodexide in its treatment. As part of an active approach to the treatment of CVI, including oedema and trophic changes in the legs, Sulodexide, also by its anti-inflammatory effect, can help alleviate the progressive signs and symptoms of the disease at any clinical stage of the CEAP CVI, from C1 to C6 [24, 29].
Sulodexide, a highly purified mixture of glycosaminoglycans composed of heparin (80%) and dermatan sulfate (20%), exhibits anticoagulant and profibrinolytic properties, also restoring the balance of the microcirculation endothelial glycocalyx. Sulodexide glycosaminoglycans have also been observed to reduce the release of inflammatory cytokines/chemokines and inhibit the proteolytic cascade associated with matrix metalloproteinases, thus counteracting endothelial dysfunction. The pleiotropic action of Sulodexide has attracted interest in its use in the treatment and prevention of CVD spectrum diseases. Many researchers pay attention to its multidirectional anti-inflammatory effect [18–24, 29, 30].
It is believed that research on metabolic changes, miRNA regulation, modulation of inflammation and glycocalyx in the pathophysiology of CVD and CVI may provide key responses for the treatment and prevention of these chronic diseases [20, 21].
Aim
Assessment of the concentration of selected acute phase proteins: CRP and AAT in the blood serum of patients with CVI before and after treatment with Sulodexide.
Material and methods
The study covered 88 people, including:
the reference group consisting of 39 people aged 29–81 years, x 51.64 ±13.94 years, clinically healthy, including 25 women and 14 men (Table 1);
the group of patients consisting of 49 people aged 26–83 years, x 52.59 ±14.07 years with diagnosed chronic venous insufficiency in stages C2 to C6 according to the CEAP scale, including 33 women and 16 men treated with Sulodexide (Tables 1 and 2).
Patients participating in the study were patients treated at the Department of Dermatology, Paediatric Dermatology and Oncology Clinic of the Medical University of Lodz. The direct form of the study involved the collection of 10 ml of venous blood per clot from the patients enrolled in the study, and a similar control examination after 1-month treatment with Sulodexide. All study groups did not differ significantly in terms of age (Table 1).
Table 1
Investigated material – age (in years)
Table 2
Clinical stage of chronic venous insufficiency (CVI) in the studied group of patients according to CEAP
| Clinical stage of CVI according to CEAP | Sex distribution | Percentage (%) | ||
|---|---|---|---|---|
| F | M | Total | ||
| C2 | 1 | 0 | 1 | 2.04 |
| C3 | 22 | 4 | 26 | 53.06 |
| C4 | 6 | 5 | 11 | 22.44 |
| C5 | 4 | 4 | 8 | 16.33 |
| C6 | 1 | 2 | 3 | 6.13 |
| Total | 34 | 15 | 49 | 100 |
The research plan received a positive opinion from the Bioethics Committee of the Medical University of Lodz. All respondents were fully aware of the nature and purpose of the study and gave their voluntary consent to participate in the study.
The concentration of CRP was determined using the turbidimetric method using reagents from the Spanish company Bio Systems, and the concentration of AAT using the L1 antitrypsin Elisa Kit from Immun Diagnostic, Germany, in the reference group once, while in the group of patients twice: before treatment and after 30 days of treatment with Sulodexide.
Sulodexide in the form of Vessel Due F in capsules of 250 LSU (standard units of sulodexide) was used in the group of patients at a dose of 2 capsules twice a day (500 LSU) for a period of 1 month. Before starting the treatment, after 14 days of using the preparation, and after completing the therapy, the coagulation system and complete blood counts were performed. Attention was also paid to the patient’s history of allergies in terms of the possibility of allergy to any component of the preparation, heparin or heparin-like drugs. Patients did not use heparin or oral anticoagulants simultaneously. All patients subjected to the study had an ultrasound examination of the venous system of the lower extremities (USG Colour Doppler) and a specific degree of venous insufficiency according to CEAP by a dermatologist and after phlebological consultation.
Determination of the concentration of CRP in the blood serum
Serum CRP agglutinates latex particles coated with antibodies to the human C-reactive protein. Latex particle agglutination is proportional to serum CRP concentration and was measured by turbidimetry. The reagents of the Spanish company Bio Systems were used for the determination. The determinations were made according to the attached manufacturer’s instructions. The reading was made on the A15 apparatus from Bor Pol. The result is given in mg/ml.
AAT assay
The AAT Elisa Kit from Immun Diagnostic, Germany, was used to determine AAT. 100 μl of the previously diluted serum and the remaining reagents were successively added to the cells coated with the monoclonal antibody, according to the instructions attached to the kit. A standard curve was constructed according to the dilutions included in the kit. The result was read over 30 min at 450 nm with a correction wavelength of 620 nm. Absorbance was measured with a Pointe 1800 plate reader. The result is given in mg/ml. Total assay time was 2 h 50 min.
Results
Assessment of clinical and laboratory tests
In the studied group of patients, the degree of venous insufficiency assessed according to the CEAP scale at the level of C3 concerned 53.06% of patients, C4 – 22.44% and C5 – 16.33% of the respondents. In those stages of the CVI, the intensification of inflammatory symptoms is observed, in the form of oedema or eczema lesions of the skin of the shin. The subjects in stages C3–C5 together accounted for 91.83% of the entire group of patients with CVI.
In the group treated with Sulodexide, no abnormalities in the coagulation system and blood counts were observed after 14 and 30 days of treatment. During the treatment, no treatment-induced side effects were observed and the tolerance of Sulodexide was very good.
CRP concentration
The concentration of CRP separately in women and men and in the entire group of patients, compared to women and men, and in the entire reference group before the use of Sulodexide, were elevated and these differences were statistically significant. There were no statistically significant differences between the studied groups of women and men (Tables 3 and 4; Figure 1).
Table 3
C-reactive protein (CRP) concentration in the blood serum of healthy and CVI patients treated with Sulodexide (mg/ml)
Table 4
Statistical analysis of CRP concentrations in the blood serum of healthy and CVI patients treated with Sulodexide
Figure 1
C-reactive protein (CRP) concentration in the blood serum of healthy and CVI patients treated with Sulodexide
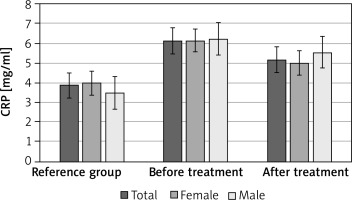
The mean concentration of CRP, separately in women and men, and in the entire group of patients after the treatment, decreased, but these differences were not statistically significant. There were no statistically significant differences between the studied groups of women and men (Tables 3 and 4; Figure 1).
AAT concentration
The concentration of AAT in the group of patients with CVI was significantly (p < 0.05) higher in women, men and in the whole group of patients compared to the reference group. There were no statistically significant differences between the studied groups of women and men (Tables 5 and 6; Figure 2).
Table 5
The concentration of α1 antitrypsin (AAT) in the blood serum of healthy people and patients with CVI treated with Sulodexide (in mg/ml)
Table 6
Statistical analysis of α1-antitrypsin (AAT) concentrations in the blood serum of healthy and CVI patients treated with Sulodexide.
Figure 2
α1-antitrypsin (AAT) concentrations in the blood serum of healthy and CVI patients treated with Sulodexide
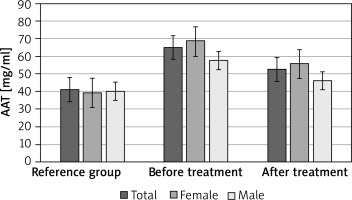
The concentration of AAT after treatment with Sulodexide decreased in all study groups, and these differences were significantly higher (p < 0.05) compared to the reference group. There were no statistically significant differences between the studied groups of women and men (Tables 5 and 6; Figure 2).
Discussion
CVD – in more advanced stages also referred to as chronic venous insufficiency or CVI – is a disease of the lower extremities with a multifactorial etiopathogenesis associated with endothelial dysfunction, inflammation, remodelling of the venous walls, valvular insufficiency, venous hypertension and reflux [22]. Other causes include obstruction of the venous outflow and failure of the calf muscle pump due to obesity or immobilization of the legs [31].
Recent studies have shown that venous hypertension and microcirculation stagnation with an accompanying change in shear stress damage the glycocalyx and induce an inflammatory response. This causes the recruitment of leukocytes through increased expression, e.g. intercellular adhesion molecules-1 (ICAM-1) and monocyte chemoattractant proteins-1 (MCP-1) and the release of pro-inflammatory factors such as interleukin-6 (IL-6), reactive oxygen species (ROS) and matrix metalloproteinases (MMP-2, MMP-9). These, in turn, contribute to tissue remodelling by promoting the degradation of extracellular matrix proteins, which results in altered structural integrity and function of the venous wall and valves [20, 22, 29, 32].
The main cause of secondary CVD is the process leading to venous hypertension, in most cases deep vein thrombosis, causing the blood flow to be narrowed at the site of the acute thrombus and the veins to widen, slowing blood flow distal to the thrombus. The complex process of acute thrombus organization and transformation with subsequent recanalization also leads to an increase in inflammatory processes. It results in progressive damage to the vein wall and valve insufficiency [33].
The relationship between inflammation and venous thrombosis is not well understood. Galeano-Valle et al. [34] believe that the inflammatory response may be both a cause and a consequence of venous thromboembolism (VTE). In fact, several VTE risk factors (obesity, surgery, sepsis, cancer) can modulate thrombosis through inflammatory markers such as CRP and P-selectin. However, their prognostic ability has not yet been precisely determined and requires further research [34].
Bignamini and Matuška [21] in a large systematic review of databases and meta-analysis of 64 studies, 23 of which provided studies of 7153 patients with CVD treated with Sulodexide, showed that Sulodexide reduced the intensity of symptoms in the form of pain, cramps, leg heaviness, oedema and decreased the activity of mediator inflammation in these patients. The risk of adverse events did not differ between Sulodexide and placebo or heparan sulfate (RR = 1.31, 95% CI: 0.74–2.32; I2 = 0%; 270 participants). The overall risk of Sulodexide-related adverse events was low: 3% (95% CI: 1–4%) estimated from screening of 3656 participants.
The authors of the study concluded that Sulodexide has a beneficial venoactive effect and may exert a systemic effect on the course of CVD by interfering with the inflammatory mechanisms related to the action of chemokines [26].
Inhibition of oxidative stress in hyperglycaemia was observed in in vivo studies in patients with diabetic nephropathy chronically treated with sulodexide for albuminuria [35].
Manello et al. found that the basis of CVD pathogenesis is inflammation within the venous microcirculation, which, when subjected to increased hydrostatic pressure, results in an increase in ambulatory venous pressure (AVP) [23], and the developing chronic inflammation includes leukocytes, in particular, macrophages and monocytes, modulators and chemokines of inflammation, pro-inflammatory cytokines, growth factors, metalloproteinases (MMPs) and many other regulatory pathways that may lead to its fixation [23].
These authors hypothesized that Sulodexide may exhibit anti-inflammatory properties by reducing the secretion of inflammatory mediators from lipopolysaccharide-stimulated (LPS) macrophages. They assessed the effect of Sulodexide on bacterial LPS stimulated macrophage secretion of various inflammatory and anti-inflammatory cytokines, chemokines and colony stimulating factors using microplate-based immunoassays. In vitro LPS stimulated macrophages caused significant increases in interleukins, tumour necrosis factor, interferon, chemokines and colony stimulating factors. The addition of Sulodexide resulted in both a dose-dependent and a dose-independent decrease in almost all inflammatory cytokines, chemokines, and colony stimulating factors. These results suggest that Sulodexide has a significant effect on the release of inflammatory mediators from macrophages and may be useful in the treatment of early and advanced stages of CVD [25].
Blomgren et al. [36] found an increase in the systemic levels of activity of the plasminogen activation inhibitor (PAI-1) and tissue plasminogen activator (tPA) with progressive skin lesions in patients with CVI, and in the group with active ulceration, an increase in prothrombin fragments 1 and 2 (F1 + 2), however, no increased concentration of CRP was found. These results may reflect the defect of fibrinolysis, thrombotic potential or endothelial damage in patients with CVI [36].
In the studies by Krieger et al. [37] the level of CRP turned out to be related to the degree of venous dysfunction in patients after acute deep vein thrombosis. Chronic inflammation and changes in blood rheological parameters may be causally involved in the development of mid- and long-term chronic venous insufficiency following acute deep vein thrombosis [37].
In the available studies, there are few reports on the role of acute phase proteins in the development of inflammation in the course of CVI, which prompted us to evaluate the behaviour of selected acute phase proteins before and after treatment with Sulodexide in patients with chronic venous insufficiency.
In our own studies, the concentration of CRP in both women and men and in the entire group of patients before the use of Sulodexide, compared to the results in the reference group, was increased and these were statistically significant differences.
The mean concentration of CRP, separately in women and in men, and in the entire group of patients after the treatment, decreased, but these differences were not statistically significant. There were no statistically significant differences between the studied groups of women and men.
The concentration of AAT was slightly different, which in the group of patients with chronic venous insufficiency was significantly (p < 0.05) higher in women, men and in the whole group of patients compared to the reference group; there were no statistically significant differences between the studied groups of women and men. However, after treatment with Sulodexide, the concentration of AAT decreased in all study groups, and these differences were statistically significant (p < 0.05) compared to the reference group. There were no significant differences between the studied groups of women and men.
Conclusions
Increased concentration of acute phase proteins: CRP and AAT in patients indicates the participation of the inflammatory component in the pathogenesis of CVI.
Monitoring levels of acute phase protein, especially AAT, may be useful in tracking the course of the disease, the body’s response to treatment, and in making prognosis.
Sulodexide, which acts mainly as an anticoagulant and profibrinolytic, also has an anti-inflammatory effect, which may contribute to inhibiting the development of subsequent stages or slowing down the development of CVI.








