Introduction
Due to common transmission routes, human immunodeficiency virus (HIV) infection may be accompanied by hepatotropic virus infections such as hepatitis B virus (HBV) or hepatitis C virus (HCV). According to the World Health Organization (WHO) there are 37.9 million people infected with HIV [1] and 257 million people infected with HBV [2] worldwide. It is estimated that, depending on the geographical region, the prevalence of HBV infection in HIV-positive patients ranges from 5% to 30% [3] and there are approx. 6 million people with HIV/HBV co-infection [4]. In comparison, HBV infection in Poland occurs in about 1% of people without HIV infection [5]. In addition, hepatitis D virus (HDV) infection is more common in HIV/HBV co-infection than in HBV mono-infection – 1.9-50% [6] vs. 2-17.3% [7]. HBV infection in HIV-positive patients does not cause progression of HIV infection and does not affect the effectiveness of combined antiretroviral therapy (cART) [8], but it is associated with a negative effect on liver function. HIV/HBV co-infection is associated with an increased risk of liver cirrhosis or hepatocellular carcinoma (HCC) and death [8]. HIV infection in HBV-infected individuals increases the HBV replication rate and the risk of developing chronic hepatitis [8]. In addition, low alanine aminotransferase (ALT) values are observed in individuals with HIV/HBV co-infection, which may hinder the diagnosis of infection on screening, despite the histological progression of hepatitis [8]. Currently recommended antiretroviral therapy (ARV) for people with co-infected HIV/HBV inhibits the replication of both of these viruses simultaneously. In the drug set it is recommended to use one of the nucleoside reverse-transcriptase inhibitors, i.e. lamivudine (3TC) or emtricitabine (FTC) with the nucleotide reverse-transcriptase inhibitor tenofovir in the form of disoproxil (TDF) or alafenamide (TAF). The aim of this study was to analyse the impact of cART on selected markers of HBV infection, as well as to assess the degree of liver fibrosis in patients on antiretroviral therapy.
Material and methods
This single-centre retrospective-prospective study evaluated patients who had been treated with ARV in the Clinic of Acquired Immune Disorders in WSSz Bieganski in Lodz, and who were found to have HBsAg. The examination included a number of selected biochemical, virological, immunological and elastographic parameters and a review of medical records. No new HBV infections were observed during ARV; HIV/HBV co-infection in the study group was diagnosed only before inclusion in cART.
The analysis was carried out in two stages. In the first, the following parameters were evaluated in all HIV/HBV infected persons treated with ARV at the time of diagnosis and over the period June-October 2018: gender, age, patterns of HIV infection, presence of anti-HCV, antiHDV, quantitatively HBV DNA viral load, CD4 count, HIV RNA viral load and serum ALT activity. In the second stage, the following parameters were determined in patients with HIV/HBV co-infection who had received ARV treatment for at least 12 months: gender, age, length of ARV treatment, CD4 number, ALT, aspartate aminotransferase (AST) and γ-glutamyl transpeptidase (GGTP) serum activity, platelet count, α-fetoprotein (AFP), liver fibrosis assessed by SWE elastography and the qualitative and quantitative presence of HBsAg.
Statistical analysis
In order to assess whether there is a correlation between HBsAg level and duration of antiretroviral treatment Spearman’s rank correlation test was used. Quantitative data are presented as median together with quartile IQR. Minimum and maximum values are also given. Qualitative data are presented as the number and percentage of patients in whom a given variable was found.
Results
Evaluation of the entire study group (stage 1)
Among the 515 patients currently receiving ARV treatment, 28 were found to have HBsAg at the time of treatment initiation (5.4%). This group included 26 men (92.9%) and two women (7.1%). The most common route of HIV infection was injection by drug users (IDU) – n = 15 people (53.6%). Men who have sex with men (MSM) constituted a group of 7 people (25%). Infection occurred through heterosexual contact (HTX) in five people (17.8%), while the route of infection was unknown in one person (3.6%). The characteristics of the study group at the time of treatment initiation are presented in Table 1.
Table 1
Characteristics of the study group at the time of treatment initiation
Evaluation of HCV co-infection in HIV/HBV infected persons
The presence of anti-HCV antibodies was found in 14 subjects (50%) – 12 men (85.7%) and 2 women (14.3%) before the start of ARV treatment. None of the subjects developed anti-HCV antibodies during the treatment. The median age of the groups was 42.5 years (IQR 12). In total, 85.7% were infected by IDU, 14.3% were MSM. At the time of the study (June-October 2018), 12 people were free of HCV RNA in serum; of these five had received successful treatment for HCV infection, seven had demonstrated spontaneous elimination of HCV. In contrast, HCV RNA was found in two people, neither of whom had previously been treated for HCV infection.
Evaluation of HDV co-infection in HIV/HBV infected persons
In June 2018 (± three months) anti-HDV antibodies were observed in six people (21.4%), including five men and one woman. The median age of this group was 43.5 years (IQR 7). None of these people had HDV RNA determined. In this group, five (83.3%) people who were anti-HDV positive were additionally found to have anti-HCV. 83.3% of those with anti-HDV were infected by IDU. All these people were of Polish nationality. One patient, a man of Ukrainian nationality, was infected by the MSM route.
Evaluation of the group of patients treated with antiretroviral therapy for at least 12 months (stage 2)
Twenty-three of the 28 HIV/HBV infected patients, i.e. 21 men and two women, had been treated with ARV for more than 12 months. The median age of this group was 44 years (IQR 12) and the median duration of ARV treatment was 80 (IQR 86) months. ARV treatment was found to demonstrate full efficacy (HIV viral load < 20 copies/ml) in 21 patients (91.3%), while the HIV viral load was 20-200 copies/ml in two patients (8.7%). HBV DNA viraemia below the detection threshold was found in 22 out of 23 patients. A comparison of selected parameters in patients treated with ARV is shown in Table 2.
Table 2
Selected parameters of HBV infected people treated with antiretroviral therapy for at least 12 months at the time of the 2018 evaluation
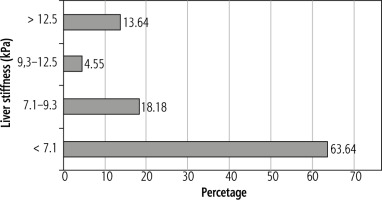
Figure 1 shows the severity of liver fibrosis by grade according to Metavir in patients treated with antiretroviral therapy for a minimum of 12 months.
HBs antigen
Six out of 23 people (6/23, 26.1%) treated with ARV for over 12 months eliminated HBsAg. Otherwise, HBsAg levels were above 1000 IU/ml in eight patients and below 1000 IU/ml in nine. An analysis of selected parameters in patient groups according to HBsAg concentration is presented in Table 3. Of the six patients who eliminated HBsAg, anti-HBs were found in three cases (3/6, 50%). A comparison of selected parameters in the patient group related to HbsAg loss is presented in Table 4. The protective level of anti-HBs is > 10 IU/l.
Table 3
Characteristics of patient groups depending on HBsAg antigen concentration
Table 4
Characteristics of patient group according to HbsAg loss
Discussion
Among the HIV patients under the care of the Lodz Centre, 5.4% demonstrated HBV co-infection. In Western Europe and developing countries chronic HBV infection is typically observed in 6-14% of HIV-positive people and is most common in the MSM group, where it is 9-17% [9].
A slightly lower incidence was observed in the present study; however, our present findings are similar to those reported by Konopnicki et al. [3]. In addition, over half of our group (53.6%) had been infected by IDU; this value is more than 1.8 times higher than that observed among HIV patients without HBV co-infect.
According to the recommendations of the Polish AIDS Society (PTN AIDS), patients with HIV/HBV co-infection should use a scheme based on TDF or TAF with FTC or 3TC. The desired treatment effect is elimination of HBsAg followed by seroconversion to anti-HBs. The 2018 recommendations of the Polish Association of Liver Study regarding the treatment of chronic hepatitis B estimate 1-3% spontaneous seroconversion in the HBs/anti-HBs system per year for cases of monoinfection [10]. Boyd et al. [11] reported that in patients treated with nucleotide analogues, the loss of HBsAg occurs at a frequency of 1-2% of patients per year. Probably seroconversion in the HBsAg/anti-HBs system will be lower in the HIV infected group because of impaired host immunity [12].
Seroconversion in the HBs system eliminates serious complications [8], and is associated with regression of inflammation and liver fibrosis [11, 13].
All patients with HIV/HBV co-infection apart from one received ARV therapy based on the above-mentioned schemes. One subject was treated with TDF alone, two with TAF alone, and 18 subjects received both TDF and TAF throughout their treatment history. One patient received treatment initially based on the 3TC/TDF regimen; however, 3TC was withdrawn after five years due to acquired resistance and the patient has received a TDF-based regimen for three years. The patient eliminated HBs antigen during ARV treatment, but he did not produce anti-HBs antibodies. Among those who lost HBsAg, one was treated with TDF alone and four with TDF and TAF. All but one patient demonstrated HBV DNA below the detection threshold. This patient demonstrated high adherence and undetectable HIV viral load, but had numerous interruptions in therapy in the past. He is currently waiting for the result of HBV genotyping.
In our group of 23 patients, with a median treatment time of six years and eight months, HBsAg was undetectable in six. Three people developed antiHBs antibodies. The disappearance of HBsAg was more common in patients receiving longer treatment, and HBsAg levels correlated with the duration of antiretroviral therapy (Rho = –0.352542, p = 0.0495). Among the patients who eliminated HBsAg, two had anti-HCV present but had eliminated HCV spontaneously. No anti-HDV were detected in any of the individuals. This participants were treated with various antiretroviral drugs, including the recommended TDF or TAF with FTC or 3TC. One patient who had built up resistance only received one of the recommended medications, and another had a four-year break in antiretroviral therapy. None of the patients in this group had previously received interferon treatment. Unfortunately, it is unclear whether antiretroviral therapy had been started at an early or late stage of HBV infection in this group, and it seems that the moment of starting treatment may play a crucial role in eliminating HBsAg in a large proportion of the study group.
For patients, HBsAg levels had prognostic significance. The gradual decrease in HBsAg is a good indicator of the effectiveness of therapy, while the persistence of HBsAg at a constant level indicates ineffective suppression of replication [8]. In the untreated group, a relationship can be seen between HBsAg levels and the occurrence of active hepatitis and cirrhosis, as well as elevated ALT activity [14]. Unfavourable levels of HBsAg are considered to be > 1000 IU/ml [14, 15].
Many authors [16-18] have reported a decrease in liver fibrosis and a lower risk of HCC in people with inhibited HBV replication during treatment with nucleotide analogues. However, HCC can occur even in patients with low HBV viraemia, and HBsAg level is a risk factor for HCC in this group. Tseng et al. [16] report that HBsAg ≥ 1000 IU/ml is an independent factor for HCC development in patients with HBV DNA < 2000 IU/ml. In the present study, eight patients co-infected with HIV/HBV had HBsAg > 1000 IU/ml and only one had HBV viraemia > 2000 IU/ml.
In the group we examined, four patients demonstrated level F4 fibrosis on the Metavir scale – three patients had been treated for more than 12 months and one patient for less. However, no patient was diagnosed with decompensated liver cirrhosis or HCC. Patients with cirrhosis had HBsAg levels between 6.52 IU/ml and 11840 IU/ml. Current PTN AIDS guidelines regarding HCC screening for patients with HIV/HBV co-infection recommend abdominal ultrasound screening and AFP assessment in all patients, regardless of the severity of fibrosis or HBsAg level [19]. In the present study, 47.8% of patients would be qualified for the screening, i.e. those who had been successfully treated, with HBV viraemia below the detection threshold, and demonstrated advanced fibrosis (≥ 3 grade in elastography) or HBsAg > 1000 IU/ml.
Another factor that negatively influences the course of HBV infection is HDV co-infection. The HDV virus is a “defective” virus that can only develop in the presence of HBsAg. HDV co-infection or superinfection is associated with poorer prognosis, faster disease progression, and with higher incidence of HCC. Particularly aggressive HBV/HDV co-infection can be observed in people infected with HIV [8]. In addition, HBsAg-positive patients co-infected with HDV demonstrate accelerated hepatic fibrosis, resulting in faster liver damage, irrespective of HIV infection, and a higher risk of developing cirrhosis than in patients with HBV alone [20].
The incidence of HDV infection ranges from 1.9% to 50% among patients with HIV/HBV co-infection [6].In the present study, anti-HDV was observed in six out of 28 patients, i.e. 21.4% of patients with HIV/HBV co-infection. However, due to the lack of availability, the diagnosis was not expanded with HDV RNA determination. The patients with anti-HDV present tended to demonstrate higher levels of fibrosis in elastographic examination; however, the study groups are too small for reliable analysis. It is worth noting, however, that cirrhosis was found in one patient out of four with anti-HDV present.
Conclusions
To summarise, in the presented group of patients, HIV/HBV co-infection occurred most often in those who had acquired it through IDU. The presence of anti-HDV was also associated with IDU acquisition of HIV. The patients with HIV/HBV co-infection demonstrated high adherence to ARV therapy; this resulted in effective HIV therapy in over 90% of patients, and effective control of HBV infection with elimination of HBsAg antigen in over 25%, half of whom produced anti-HBs antibodies.
The authors received the approval of the Bioethics Committee for the research, approval number: RNN/223/19/KE.