Introduction
Atopic dermatitis (AD) affects 10–20% of children worldwide and persists into adulthood in a minority of cases, affecting approximately 2–3% of the adult population, with an increased prevalence over the past decades in developed countries. Atopy is a genetic tendency to overproduce IgE class antibodies in response to common antigens found in the environment. Concurrence of different atopy such as allergic rhinitis or asthma in children with AD is estimated at 80% [1, 2]. AD is characterized by a vicious cycle of an allergic immune response. Disrupted skin barrier due to biological or genetic factors, induces production of inflammatory cytokines from keratinocytes, which enhances the allergic immune response. Itch induced by the allergic immune response triggers scratching, which exacerbates skin barrier dysfunction. Pruritus is a major symptom of AD which impacts the quality of patient’s life to a great extent. Patients with severe or persistent disease and their families experience significant impairment in their quality of life [3], and in addition, AD places a heavy economic burden on the society as a whole [4].
Previously there were two major competing hypotheses regarding the pathogenesis of AD. The inside-out hypothesis which stated that AD is a result of the imbalance in the adaptive immune system that affects the skin barrier by creating a cutaneous inflammatory state and leads to its impairment. In this theory, barrier disruption is caused by a number of factors such as itching, caused by highly expressed IL-31RA, which leads to scratching [5] and thus, higher penetration of allergens, more abundant Staphylococcus aureus presence caused by immune imbalance [6], and downregulated filaggrin production in the skin [7]. More recently, AD has been linked to mutations and polymorphisms in IL-10 and IL-13 genes [8], which also strengthened this theory.
The outside-in hypothesis suggested that skin barrier abnormalities occur first and trigger dysregulations in the immune system. This theory was strongly supported by a number of studies linking, on average, 20% of AD cases in Europe and Asia with null mutations in filaggrin gene FLG [9–11]. However, it is important to state that in Africa AD affects 15% of children [12] while less than 5% of residents of African descent have a mutated filaggrin gene. Filaggrin is paramount for proper function of the skin barrier because of its role in the release of the natural moisturizing factor (hygroscopic amino acids) and in the creation of cornified cell envelope during cornification of keratinocytes [13]. Therefore, according to this hypothesis, unfunctional filaggrin leads to the disrupted skin barrier resulting in cutaneous inflammation caused by Th2 cells [14].
Over time it became clear that the pathophysiological concepts underlying the development of AD are multi-factorial and include activation of different lymphocyte subsets, multi-cytokine immune dysregulation, epidermal barrier disruption, and microbiome imbalance. Skin barrier disruption or immunologic abnormalities may be more abundant at first in one AD patient than in another, but it is clear that those irregularities are simultaneously responsible for pathogenesis of atopic dermatitis [15].
Acute and chronic atopic dermatitis
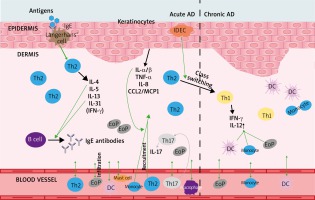
Atopic dermatitis is a common allergic skin disorder characterized by a chronic relapsing form of skin inflammation, disturbance of epidermal barrier function that leads to dry skin, and keratinocyte apoptosis as a mechanism of eczema and spongiosis formation, which is mostly seen in acute and subacute lesions. The pathogenesis of AD is multifactorial, including genetic, environmental, skin barrier, physiological and immunological factors. Acute AD skin lesions are intensely pruritic, erythematous papules associated with excoriation and serous exudation. There is a marked infiltration of CD4+ T cells, CD8+ T cells, eosinophils, mast cells and dendritic cells [1]. Chronic AD skin lesions are characterized by tissue remodelling caused by chronic inflammation. The skin lesions are associated with thickened plaques, increased collagen deposition in the dermis, and dry fibrotic papules. Macrophages dominate the dermal mononuclear cell infiltrate. Eosinophils also contribute to the inflammatory response and T cells remain present, although in smaller numbers than seen in acute AD [1, 2, 16]. During AD, resident structural elements of the skin (keratinocytes, fibroblasts, endothelial cells) closely interact with cells that are actively recruited from the blood in response to inflammatory stimuli. Complex cytokine and chemokine environment controls recruitment of T cells from the blood vessels into the skin. During the early stages, keratinocytes produce cytokines and chemokines after mechanical trauma and skin barrier disruption [1, 2, 16]. This is reflected clinically by the itch-scratch cycle, which may represent the initial point for starting an inflammatory reaction. Keratinocytes start to produce IL-1α/β, TNF-α, IL-8 and CCL2/MCP-1. This leads to infiltration of T cells and monocytes and production of further keratinocyte-derived chemokines and cytokines. The local response of keratinocytes together with a reaction of endothelial cells, T cells, mast cells, macrophages, eosinophils, and dendritic cells finally leads to the characteristic clinical and histological appearance of AD. The generalized Th2-deviated immune response is usually linked to AD, however, the skin disease itself is a biphasic inflammation with an initial Th2 phase and a chronic Th1 phase [1, 16]. A predominant systemic Th2 cytokine response with increased IgE levels and eosinophilia is widely accepted as a part of the AD pathogenesis. IL-4, IL-5, IL-13, and IL-31 are major Th2 cytokines that participate in AD and in other allergic disorders. IL-4 and IL-13 have essential roles in the initial phase of tissue inflammation and in the increased expression of adhesion molecules on endothelial cells [2, 16]. Both cytokines are also responsible for the differentiation of allergen-specific Th2 cells and the class switching of activated B cells to IgE-producing cells. IL-5 contributes to the increase and survival of eosinophils [2, 16]. The epidermis with atopic inflammation contains 2 distinct types of dendritic cells including the classical Langerhans cells and the inflammatory dendritic epidermal cells [17]. The latter population induces Th1 cytokines, whereas the Langerhans cell population induces Th2-type reactions [18]. The balance of these populations may induce Th2/Th1 cytokine profile switching characteristic of the chronic phase of AD. Acute AD skin lesions are characterized by T cells and eosinophils with deposition of eosinophil-derived products and increase skin expression of Th2 cytokines IL-4, IL-5, IL-13 and IL-31 with only little interferon-γ (IFN-γ) expression [16, 17, 19]. Accumulation of activated monocytes, mature dendritic cells, and eosinophils determines a rise in IL-12 expression, appearance of Th2/Th1 switch and the presence of IFN-γ in chronic AD lesions [19]. The processes occurring in acute and chronic AD are presented in Figure 1.
Apoptosis in atopic dermatitis
Upregulation of Fas by IFN-g sensitizes keratinocytes to Fas-induced apoptosis by invading T cells with FasL expression [20]. IL-17 is an important component of allergic and inflammatory diseases. Infiltration of IL-17-producing T cells (Th17) is known to be also abundant in the acute AD skin lesions [21]. IL-17-producing T cells (Th17) are distinct from Th1 and Th2 cells and have roles in innate and adaptive immunity [22]. IL-17 is involved in inducing and mediating pro-inflammatory responses through up-regulation of IL-8, CXCL10 and TNF-α production by keratinocytes and fibroblasts [22]. The chemokines that recruit Th2 cells into inflammatory sites have been categorized as Th2 chemokines. Chemokines also control Th1 cell chemotaxis. CCL20/MIP-3a is an important Th1 chemokine responsible for the recruitment of CCR6-expressing immature dendritic cells and memory/effector T cells into the dermis of atopic skin through CCR6 [23]. On the other hand, CXCL9, CXCL10, and CXCL11 recruit mainly Th1-type lymphocytes to the inflammatory sites [24]. Several studies have demonstrated that keratinocyte apoptosis is an important component of eczema and spongiosis in patients with AD and is mediated through Fas/FasL.
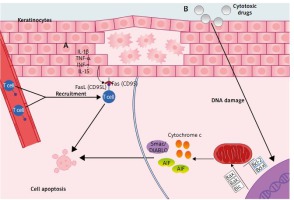
Two major apoptosis signalling pathways have been discovered: receptor-ligand mediated pathway (extrinsic) and mitochondrial-driven pathway (intrinsic). These pathways are regulated by a number of pro- and anti-apoptotic molecules. The extrinsic pathway is initiated by ligand binding to cell-surface death receptors (tumour necrosis factor receptor) superfamily (i.e. Fas, tumour necrosis factor, and tumour necrosis factor related apoptosis-inducing ligands: (TRAIL)-R1 and TRAIL-R2) [25]. Signalling via death receptors plays a crucial role in the activity of the immune system, where it contributes to the regulation of the adaptive immune response in various ways, most notably by triggering apoptosis of T cells [25]. Fas (CD95) receptor activates the apoptosis pathway upon binding to its physiological ligand FasL (CD95L). Fas is expressed on a variety of both lymphoid and non-lymphoid cells (e.g. liver, ovary, heart, lung, kidney, and skin). In contrast, FasL expression is limited to a few cell types (e.g. activated lymphocytes, some keratinocytes). The ‘intrinsic’ pathway involves a mitochondrial release of e.g. cytochrome c, AIF – apoptosis inducing factor, smac/DIABLO, which are released into the cytoplasm upon apoptosis induction triggered by cytotoxic drugs and DNA damage. The release of these pro-apoptotic factors is regulated by Bcl-2 family proteins forming homodimers and heterodimers that control mitochondrial membrane permeability. Some Bcl-2 family proteins (Bcl-2 and Bcl-xL) may block apoptosis whereas others (Bax, Bak, and Bid) promote apoptosis by interfering with these interactions [26]. Extrinsic and intrinsic apoptotic pathways are presented in Figure 2.
Figure 2
Apoptosis of keratinocytes in AD: A – Extrinsic (receptor-ligand mediated pathway), where stimulation of the keratinocytes by pro-inflammatory cytokines triggers the upregulated Fas receptor expression, while T cells are recruited from the blood vessel. FasL induces apoptosis through binding with FAS receptor. B – Intrinsic (mitochondrial-driven pathway), where apoptosis is induced by cytotoxic drugs and/or DNA damage, which stimulates mitochondria to release pro-apoptotic factors. This process is regulated by Bcl-2 family proteins; AIF – apoptosis-inducing factor
In normal skin, terminal differentiation of keratinocytes consists in a special form of apoptosis (cornification) which shows similarities between terminally differentiating keratinocytes and apoptotic cells; for example, granular keratinocytes show signs of endonuclease activation and DNA fragmentation [27]. Apoptosis in the skin represents a key event of the epidermal homeostasis. Its removes exceeding cells and guarantees the normal epidermal architecture, represents an important anticancer defence mechanism in response to UV radiation or oxidative damage [27]. Epidermal keratinocytes express Fas and FasL in low amounts [28]. Abnormal expression of lytically active FasL was found in inflammatory skin diseases such as toxic epidermal necrolysis, atopic dermatitis and allergic contact dermatitis [29]. After stimulation with pro-inflammatory cytokines such as IL-1 β, tumour necrosis factor α (TNF-α), IFN-γ and IL-15, but not IL-10, IL-12, TGF-β, keratinocytes express in a time- and dose-dependent manner FasL [29]. Furthermore, there are several ways to trigger apoptosis in keratinocytes [29]: detachment of cells from tissue, triggering of the Fas receptor, activation of the perforin/granzyme B pathway, induction of genomic DNA damage, intracellular generation of ceramides catalysed by sphingomyelin hydrolase.
Role of T cells in keratinocyte apoptosis
Activated cytotoxic FasL+ T-cells are able to kill Fas+ keratinocytes. Cytotoxic T cells and Th1-cells express both FasL and perforin, while Th2-cells do only express FasL. Cytotoxic T cells mainly use the perforin pathway, while Th2-cells act through FasL and Th1-cells inconstantly use both possibilities [25]. As mentioned, keratinocyte apoptosis is an important component of eczema and spongiosis in patients with AD [29]. Furthermore, IFN-γ-induced apoptosis occurs through Fas both in keratinocytes and IFN-γ-producing T cells. Although IFN-γ appears to be a key factor, other cytokines, such as TNF-α, TNF-like weak inducer of apoptosis (TWEAK), and IL-32, can also contribute to keratinocyte apoptosis in patients with AD [30, 31]. Emerging evidence indicates that Fas/FasL death receptors activate inflammatory or proliferative signalling via the prototypic proinflammatory transcription factor NF-κβ or the mitogen activated protein kinase (MAPK) family of kinases [32]. Farley et al. demonstrated that FasL triggered an NF-κB-dependent mRNA accumulation of inflammatory cytokines (TNF-α, IL-6, and IL-1β), chemokines (CCL2, CXCL1, CXCL3, and CXCL8/IL-8), and the adhesion molecule ICAM-1 in HaCaT cells and in reconstructed human epidermis (RHE) [33]. Activation of Fas was required both for apoptosis and for gene expression. Inhibition of caspase activity abolished FasL-dependent apoptosis; however, it failed to suppress the expression of FasL-induced genes [34]. Additionally, in the presence of caspase inhibitors, but not in their absence, FasL triggered the accumulation of CCL5 (regulated on activation normal T cell expressed and secreted) mRNA. Furthermore, Farley et al. found that FasL-stimulated autocrine production of epidermal growth factor receptor (EGFR) ligands, and the subsequent activation of EGFR and ERK1 and ERK2 mitogen-activated protein kinases, were obligatory extracellular steps for the FasL-induced expression of a subset of inflammatory mediators [35]. Also, Krzyżowska et al. showed that murine keratinocytes stimulated with Fas cytotoxic antibody start to produce TNF-α, CXCL10 and IL-1β [36, 37]. It is known that skin cell death receptors are expressed, but there is no evidence of extensive apoptosis of these cells, suggesting that non-apoptotic mechanism of Fas/FasL pathway is a commonly encountered phenomenon, although it has not been examined in the case of atopic dermatitis. Moreover, Farley et al. have previously shown that FasL induces production of secondary cytokines which trigger in keratinocytes the following inflammatory response – this indicates the existence of an autoactivation loop of cytokines in the skin [33]. Thus, the Fas/FasL pathway may have a wider range of functions in the skin than previously suspected and may act as a potential starting point for further cutaneous inflammation.
Cytokines levels in atopic dermatitis have a significant impact on the course of the disease. Therefore, we shortly review the most important cytokines and their role in the pathogenesis of AD.
Th1 cytokines
IL-12
IL-12p70 cytokine is a heterodimer consisting of two subunits p35 and p40. It is produced by dendritic cells, macrophages, granulocytes, mast cells, and keratinocytes. IL-12p70 stimulates the proliferation, activation and cytotoxicity of T cells, and NK cells, as well as the production of cytokines such as IFN-γ and TNF. In the chronic phase of AD, upregulated secretion of IL-12p70 is responsible for modulating the immune and inflammatory response, by stimulating the differentiation of Th0 cells into Th1-type [2, 38, 39].
TNF
TNF-α is one of the major immune response cytokines, primarily produced by not only stimulated monocytes and macrophages, but also keratinocytes [40]. The primary function of TNF is the regulation of the immune cells. It enhances the cytotoxicity of macrophages and monocytes, activates neutrophils and has chemotactic properties. TNF stimulates the production of reactive oxygen species and various cytokines (e.g., IL-1 and IL-6), induces inflammation and inhibits oncogenesis. TNF is an activator of the NF-κβ pathway and is itself regulated by this pathway [41, 42]. In atopic dermatitis, TNF-α together with IL-4, IL-13, and IL-31 induce intercellular oedema and promotes allergic inflammation in the skin. In addition, increased levels of TNF-α along with IL-31, in the atopic skin, affect the structure and composition of lipids [43].
Th2 cytokines
IL-4
IL-4 is a Th2-type cytokine primary produced by mitogen- or antigen-stimulated helper T-cells, natural killer T-cells, mast cells and basophiles. Scientific reports suggest that keratinocytes also produce IL-4, and dermatitis causes an increase in its production [44, 45]. IL-4 has many important functions, inter alia, induction of allergic reactions, stimulation of B lymphocytes proliferation and IgE production, as well as induction of T-cells differentiation into Th2-type. IL-4 has a significant role in maintaining skin homeostasis and is inversely correlated to the production of the EDC gene that regulates the function of the epidermal barrier [46].
IL-13
IL-13 is an immunoregulatory Th2-type cytokine secreted primarily not only by stimulated Th2 lymphocytes, but also by CD8+ T cells, NK cells, mast cells and keratinocytes [44, 47]. IL-13 acts primarily on monocytes and stimulates the proliferation of activated B-cells. Regulation of the synthesis and release of IL-13 by the cells is complex and depends on the influence of IL-4, IL-12, IL-18, IFN-γ, IL-10, TGF, and TNF-α [48]. IL-13 is a key mediator of skin inflammation which results in dysfunction of the epidermal barrier: its concentration is significantly higher in the atopic skin compared to healthy skin, and elevated levels of IL-13 and IL-4 increase susceptibility to skin infections caused by Staphylococcus aureus [49–51].
IL-31
IL-31 is a cytokine from the IL-6 family primarily produced by activated Th2 type T cells. Binding of IL-31 to the cell surface receptor leads to activation of the MAP kinase and PI3K/AKT pathway. Depending on the concentration of the cytokine and cell type affected, IL-31 stimulates or inhibits cell proliferation. Furthermore, IL-31 may increase the secretion of proinflammatory cytokines and chemokines, as it happens in AD keratinocytes. Upregulated expression of proinflammatory cytokines and chemokines leads to the recruitment of T lymphocytes. Interestingly, a high expression of IL-31RA is observed on sensory neurons that are directly responsible for the itching sensation. Increased IL-31 concentrations in inflammatory areas in patients with atopic dermatitis may, as with IL-4 and IL-13, lead to increased susceptibility to skin infections caused by Staphylococcus aureus [52, 53].
Other cytokines
IL-1
IL-1β is one of the main regulators of the immune and inflammatory response, which affects almost all kinds of cells through IL-1RI. Moreover, IL-1β is a MAP kinase and NF-κβ transcriptional factor agonist, which leads to the production of IL-6 [54]. In AD, the release of IL-1β from the epidermis is the first event following allergen activation. It increases the expression of adhesion molecules on endothelial cells, which facilitates the formation of proinflammatory and immunocompetent cells infiltration [55].
IL-10
IL-10 is an anti-inflammatory cytokine, mainly produced by stimulated T cells, B lymphocytes, macrophages, monocytes, and keratinocytes. It inhibits the cell-type immune response and inflammatory response. However, studies suggest that IL-10 also has immunostimulatory properties, with the function of eliminating infectious and allergic agents with limited inflammation. Numerous in vitro studies conducted in murine models and expression analysis of patients showed a significant effect of IL-10 on the course of inflammatory, cancer and autoimmune diseases [56]. Among researchers investigating keratinocytes, there is an ongoing debate about the secretion of IL-10 by keratinocytes derived from patients with AD [45, 57].
IL-8
IL-8 (CXCL8) is a proinflammatory chemokine secreted by many types of cells, including keratinocytes. The main function of IL-8 is the induction of neutrophil chemotaxis as well as the stimulation of their bactericidal properties. Furthermore, CXCL8 stimulates keratinocyte proliferation [58]. Activation of the Fas/FasL pathway results in the increased secretion of IL-8 by these cells [59, 60].
IL-17
IL-17 is a cytokine produced by activated CD4+ T-cells. It is extremely pleiotropic, affecting (stimulating) many types of cells, including epithelial cells, endothelial cells, fibroblasts, and macrophages. Also, IL-17 induces maturation of dendritic cells and is involved in activation of NF-κβ transcription factor in the target cells [61]. Furthermore, IL-17 via the TRAF4-ERK5 signalling cascade stimulates keratinocyte proliferation [62]. IL-17 plays a significant but not fully known role in AD, for example, Nograles et al. observed that IL-17 induces non-specific immune response [63]. Moreover, in the early stages of inflammation, IL-17A can induce and/or modify T-cell differentiation in the Th2 type [64]. One of the most important functions of IL-17 in atopic dermatitis is the induction of proinflammatory responses in keratinocytes by increasing the expression of GM-CSF, IL-8, and TNF CXCL10 [65].
IL-33
IL-33 is a cytokine from the IL-1 family primary secreted by damaged endothelial and epithelial barrier cells, fibroblast-like cells and skin keratinocytes [66, 67]. IL-33’s main function is to act as an alarmin – a molecule that activates the innate immune system [68]. IL-33 is exclusively recognized by the ST2 receptor, which is highly expressed on Th2 and ILC2s cells [69]. Binding of the IL-33 to ST2 receptor leads to activation of MAP kinases (ERK, JNK, and p38), and NF-κB, through the IL-1RAcP recruitment and heterodimer formation. Activation of the MAP kinases and NF-κB-dependent pathways promotes ST2 positive cells survival, proliferation and Th2 cytokine secretion (IL-4, IL-5, IL-13) [70]. In atopic dermatitis, IL-33 is released by the epithelium upon allergen exposure and leads to maturation, migration and promotes survival of the basophiles and mast cells [71]. What is more, anti-IL-33 antibody called ANB020 was shown to reduce EASI score and blood eosinophil levels in patients with moderate-to-severe adult atopic dermatitis in Phase-2a clinical trial [72].
IL-19
IL-19 is a proinflammatory cytokine from the IL-10 family, which is mostly secreted by monocytes, keratinocytes, and epithelial cells [73–75]. The main function of the IL-19 is to promote the production and secretion of Th2 cytokines. IL-19 is induced by IL-17A and is recognized by the IL-20R1 and IL-20R2 receptors and leads to activation of JAK and STAT3 pathways [76, 77]. In atopic dermatitis, IL-19 is highly expressed in the skin lesions. Interestingly, keratinocytes secrete IL-19 as well as express IL-20R1 and IL-20R2 receptors suggesting that IL-19 can act in an autocrine manner. Finally, it was shown that the IL-19 amplifies the IL-17A effects in keratinocytes [78].
Current research regarding cytokines in AD
The analysis of scientific literature, summarized in Table 1, revealed that the majority of research does not have an established and unified research methodology or research material. Results obtained from in vitro cell studies are not reflected in blood tests or biopsy samples. The most popular cytokines tested are IL-8, IL-10, IL-13, IL-19, and IL-17.
Table 1
Analysis of scientific literature regarding atopic dermatitis
| Author, year of publication | Material | Main findings | |
|---|---|---|---|
| Chen et. al., 2016 [95] | Human T helper cell line Jurkat and 293T | In Jurkat cells overexpressing miR-151a, the following was noticed: • decreased expression levels of IL-2, IL-12, and IFN-γ • downregulation of IL12RB2 which expression was regulated by miR-151a by targeting two loci in the 3’ untranslated region of the IL12RB2 gene | |
| Brunner et. al., 2017 [84] | 59 patients with moderate-to-severe AD (31 male and 28 female patients), 20 patients reported to have mild asthma, of which 11 (18.6%) were on asthma treatment (inhaler); 9 patients (16.1%) reported seasonal allergies | 10 increased proteins in serum of both diseases: IL-16, IL-17C, IL-2RA, TNF, and IFN AD patients showed increased level of: T-cell development/activation (IL-7, IL-2RB, IL-15RA) Th2 (IL-13, IL-10, IL-10RB), Th1, Th1/Th17/Th22 (IL-12/IL-23p40), and IL-17 responses in serum consistent with skin expression AD inflammatory mediators (e.g. IL-12/IL-23p40) correlated between blood and lesional and non-lesional skin In psoriasis, TNF-R2, IL-17A, IL-6, IL-1RA, and IL-1RL1 were upregulated Multiple inflammatory pathways showed stronger enrichment in AD than psoriasis Several atherosclerosis mediators in serum (e.g. IL-16) correlated with SCORAD, but not BMI Pathways exclusively enriched in AD included: cytokines and inflammatory responses, T1/T2 differentiation, dendritic cell pathway, asthma, IL-12 STAT4 signalling, CD40L signalling, IL-4 signalling, and CXCR3 signalling, IL-2 STAT5 signalling, CD8+T-cell signalling, and TCR signalling Pathways exclusively enriched in psoriasis included: HIF1-α transcription factor network, AMB2 neutrophils pathway, NK-cell mediated cytotoxicity, ceramide signaling, and innate immune system (Reactome) | |
| Weiss et al., 2017 [89] | 3 patients with severe AD who were administered 45 mg of subcutaneous ustekinumab over a period of 16 weeks | Decrease: • in IL-12/-23 p40 in dermal mast cells and epidermal Langerhans cells • in IL-13, IL-12/-23p40, IL-19, IL-22 mRNA levels after 8 weeks of ustekinumab treatment Heterogeneous expression of IFN-γ and IL-17A, IL-23 p19 mRNA level | |
| Orciani et al., 2017 [79] | Mesenchymal stem cells (MSCs) isolated from the skin of 12 adult patients (7 male and 5 female patients) with chronic AD • The control group included 12 healthy white adults (7 male and 5 females patients) | AD-MSCs showed: • upregulation of IL-3, IL-6, IL-8, IL-12B, IL-13, IL-17A, IL-17F, and IL-21 • downregulation of IL-2, IL-4, IL-5, and IL-23A • normal regulation of IL-17C and TNF • no significant differences among different samples • no significant variations between C-MSCs and AD-MSCs The results obtained by ELISA mostly reflected those found in the PCR analysis: • IL-3, IL-17F, and IL-23A were normally expressed • IL- 6, IL-8, IL-12, IL-13, IL-17A, IL-21, and TGF-β increased in AD-MSCs • IL-2, IL-4, and IL-5 were produced in lower amounts by AD-MSCs • IL-17C, TNF-α were similarly secreted by CMSCs and AD-MSCs | |
| Yang et al., 2017 [80] | 37 paired atopic eczema skin lesional tissues and normal non-lesional tissues | miR-124 expression was possibly downregulated by all the indicated inflammatory factors (IL-13, IL-1β, IL-17A, IL-4) and more strongly downregulated by IFN-γ and TNF-α IL-8 mRNA expression was upregulated in chronic lesional skin of patients with atopic eczema, and all inversely correlated with miR-124 | |
| Moy et al., 2017 [96] | Skin biopsies obtained between 2009 and 2014 (retrieved from the pathology archives) Psoriasis not treated with TNF-α inhibitors (n = 9) Psoriasis previously treated with/unresponsive to anti-TNF-α therapy (n = 11) PsoD related to antiTNF-α treatment for another underlying condition (n = 9) and AD (n = 9) | Decreased Th1: Th2 ratio and increased Th17 : Th1 ratio in anti-TNF-α-unresponsive psoriasis and anti-TNF-α-related PsoD comparing to untreated psoriasis Anti-TNF-α-unresponsive psoriasis had fewer Th1 (4% vs. 12%) and more Th17 (51% vs. 20%) cells than untreated psoriasis No difference in Th22 cells was identified K17 present in all cases of untreated psoriasis and anti-TNF-α-related PsoD, 91% of anti-TNF-α-unresponsive psoriasis, and only 22% of AD VCAM-1 and ICAM-1 in anti-TNF-α-related PsoD was akin to untreated psoriasis but decreased in anti-TNF-α-unresponsive psoriasis | |
| Stoffel et al., 2017 [86] | 19 skin biopsies from inflammatory bowel disease (n = 17) and rheumatoid arthritis (n = 2) patients with new-onset inflammatory skin lesions during anti-TNF-α – therapy | Conventional psoriasis had the highest levels of IL-17A, IL-17F, IL-19, IL-1B, within each comparison Eczema had the highest mRNA expression levels of IL-4, IL-13, IL-9 Psoriasis form and eczematous lesions showed increases in the pro-inflammatory mediators: IL-36A, IL-36G, IL-19, and IL-20, as well as the IFN-α marker Mx1, scalp lesions lacked upregulation of these mediators Eczematous lesions showed upregulation of IL-13, IL-5 and showed highest levels of IL-22 comparing to conventional psoriasis, eczema and anti-TNF-α lesions All forms of anti-TNF-α-induced lesions showed higher increases of IFN-γ and in IFN-γ regulated molecules such as the IL-12 receptor subunit IL12RB2 comparing to psoriasis and eczema | |
| Dos Santos et al., 2017 [97] | Serum from 21 patients with AD and 21 healthy non-AD volunteers | Reduced frequency of IFN-c + and TNF+ after TLR2 (Pam3CSK4) and TLR7/8 (CL097) stimuli Increased frequency of IL-10 under TLR4 (LPS) stimulation in AD individuals Increased frequency of IL-10 after TLR7/8 (CL097) stimulation in PBMC | |
| Ovsiy et al. 2017 [98] | RAW 264.7 cell line | Presence of FoxQ1 resulted in significant acceleration of TNFα secretion 3 h and 6 h after LSP stimuli in RAW264.7 cell lines LSP1 expression was not decreased in monocytes /macrophages stimulated with IL-4 for 3 h or 6 days Significant inhibition of plexin C1 expression found in human monocytes stimulated with IL-4 for 3 h and in monocyte-derived macrophages differentiated with IL-4 for 6 days | |
| 8 patients (18–60 years old) with clinically proven atopic dermatitis and high serum IgE levels Human peripheral blood mononuclear cells stimulated with 10 ng/ml IL-4 and 100 ng/ml IFN-γ | FoxQ1 expression in macrophages is stimulated by IL-4 (after 6 days) and suppressed by IFN-γ in AD patients Re-stimulation of IL-4-treated macrophages with IFN-γ or LPS resulted in rapid downregulation of FoxQ1 mRNA expression 3 h after the addition of the IL-4 and between 6 h and 25 h of stimulation expression of FoxQ1 rapidly increased in healthy donors On the protein level, FoxQ1 was weakly expressed in non-stimulated macrophages and more pronouncedly in IL-4 stimulated monocyte-derived macrophages as detected by immunofluorescent staining/confocal microscopy on day 6 of culture | ||
| Cho et al., 2017 [81] | The frozen stored serum samples from 16 adult AD patients with IgE-mediated sensitization to HDM | Increased serum level of IL-10 and IFN-γ at 4, 8, and 12 weeks compared with baseline There were no significant differences in the serum levels of IL-4 or IL-12 before and after intramuscular administration of autologous total IgG | |
| Mashiko et al. 2017 [90] | Lesional skin biopsies and blood collected from AD patients; n = 12, psoriasis; n = 11 | Infiltrating T cells produce IL-4 and IL-13 prior to stimulation in lesional skin of AD but not psoriasis Basophils that express IL-4 are detected in lesional skin of AD but not psoriasis and correlate with skin ILC2 and not blood ILC2 | |
| T cells from PBMC did not express IL-4 and IL-13 No cytokine was detected in ILCs from freshly isolated PBMC Basophils from PBMC did not express either cytokine, only IL-4 was increased following stimulation irrespective of disease examined IL-4 production by circulating basophils and Th2 cells and IL-13 by ILCs and Th2 cells was similar in both diseases | |||
| Sheikhi et al., 2017 [82] | PMBC’s from 20 AD patients (age 1–12 years) co-cultured with different concentrations of UV killed L. Bulgaricus | L. Bulgaricus significantly • up-regulated the secretion of IL-10, IL-12, and IFN-γ • decreased the secretion of IL-4 by PBMCs at both incubation times 48 h/72 h and both bacteria: PBMCs ratios 100 : 1/50 : 1. There were no significant differences between incubation times 48 h and 72 h regarding the secretion levels of IL-12, IFN-γ, and IL-4 The secretion of IL-10 by L. Bulgaricus-stimulated PBMCs at incubation time 72 h and in the presence of bacteria: PBMCs ratio 100 : 1 was significantly higher than in incubation time 48 h and in the presence of bacteria: PBMCs ratio 50 : 1 | |
| Tyurin et al., 2017 [83] | The first group composed of 50 individuals (25 people with atopic dermatitis and 25 with atopic dermatitis combined with either allergic rhinitis or allergic bronchial asthma) The control group consisted of 100 persons (medical personnel) without symptoms of atopic dermatitis | The group of patients having the heterozygous genotype for the TLR2 receptor had: • significant reduction in serum levels of INF-γ (1.5 times lower) • increase in the levels of IL-4 and IL-10 (1.4 and 1.8 times higher) The heterozygous group for the TLR4 receptor had: • significant reduction in serum levels of INF-γ (1.6 times lower) • significant increase in the concentrations of serum interleukins IL-4 and IL-10 (1.3 and 1.6 times higher) Serum levels of INF-γ significantly decreased (1.4 times lower) in patients with AD Serum levels of IL-4 and IL-10 were 1.6 times higher but only in the groups of sick individuals with heterozygous genotypes for both receptors No significant differences were detected in healthy individuals for the serum levels of cytokines depending on the genotype | |
| Czarnowicki et al., 2017 [91] | Peripheral blood from 32 moderate-to-severe alopecia areata (AA) adults with 43 moderate-to-severe AD patients and 30 age-matched controls | IL-13 frequencies were significantly higher in AD IFN-γ levels were mostly similar in AA, AD patients, and controls | |
| Guttman-Yassky et al., 2017 [85] | Biopsied lesions from 30 adult patients (16 male and 14 female patients) with mild-to-moderate AD | IL-13, and IL-22 were similarly reduced only by steroids Both steroids showed similar improvements, and only a few markers (including IL-5) showed larger reductions with clobetasol | |
| Brunner et al., 2017 [87] | 29 lesional and 19 non-lesional AD biopsies, compared to 6 healthy control | Lesional T-cell fractions correlated with IL-13, IL-23p19, and IL-21 responses Neither T-cell fraction nor clonality levels correlated with serum IgE or SCORAD | |
| Nygaard et al., 2018 [86] | Serum from 71 adults and 61 children with AD and 31 adult controls | Serum levels of IL-31 and IL-33 were significantly elevated in AD patients compared with controls In AD patients, both IL-31 and IL-33 serum levels were higher in children than in adults We observed no correlation between disease severity and any of the investigated cytokines Serum levels of IL-31 and IL-33 were affected to a small extent | |
| Esaki et al., 2017 [88] | Biopsy specimens from 19 children with AD younger than 5 years within 6 months of disease onset in comparison with adults with AD or psoriasis and paediatric and adult control subjects | In lesional skin children showed comparable to adults, strong activation of IL-13, IL-31 and IL-22 axes and IFN-γ was present Children showed significantly higher induction of IL-17A, IL-19, IL-9, IL-33, and IL-8 than adults Nonlesional skin in children with AD showed higher levels of IL-17A and IL-19 and epidermal proliferation markers | |
| Nattkemper et al., 2017 [93] | Skin from paired itchy, lesional and non-itchy, non-lesional skin biopsies from 25 atopic dermatitis (AD) and 25 psoriasis patients and site-matched biopsies from 30 healthy controls | Cytokines such as IL-17A, IL-23A, and IL-31 had elevated gene transcript levels in both itchy atopic and psoriatic skin Expression of genes for IL-10 was found to be increased only in pruritic atopic skin, while expression of genes for IL-36α/γ was elevated only in pruritic psoriatic skin | |
| Martel et al., 2017 [94] | Skin biopsies from patients with extrinsic AD (n = 6), intrinsic AD (n = 9) and psoriasis (n = 9) | Few cells were found to be positive for IL-17 and its transcription factor RORct No significant difference detected for any of the other measured transcription factors or cytokines either intracellularly or in the supernatant IL-9, IL-12, IL-21, IL-25, IL17A, IL-17F, and IL-33 could not be quantified in the supernatants of any culture IL-22 was only quantifiable in media from a single extrinsic AD culture and IL-31 in two intrinsic AD cultures, and these cytokines were not detected in any of the psoriatic cultures No differences found in the cytokine profile of T cell cultures derived from extrinsic AD and intrinsic AD skin | |
| Bernard et al., 2017 [99] | Reconstructed human epidermis (RHE) from keratinocytes of the outer root sheath of hair follicles 12 healthy individuals and 7 AD patients | IL-1β (around 90 pg/ml) promotes TSLP secretion by the healthy human epidermis and TSLP secretion in keratinocytes through the NF-κB-dependent pathway IL-1β synergizes with TLR ligands to promote RHE secretion of MCP-3, RANTES, and IL-6 IL-1β alters keratinocyte terminal differentiation of healthy RHE RHE generated from AD donors with functional FLG alleles respond to IL-1β signals in a way similar to that of healthy human RHE | |
| Sgnotto et al. 2018 [92] | Thymic tissues obtained from 14 patients who underwent corrective cardiac surgery Blood samples were collected from 10 subjects who were previously clinically classified as having moderate or severe AD | TDP cells cultured with purified IgG from AD patients, and TDP cells produced higher levels of IL-17A, IL-10, and TGF-β compared to the levels in both mock and IVIG-treated cells Higher production of IL-17A and IL-10 in TCD4 cell population after culture with AD IgG compared to mock and IVIg-treated conditions Higher levels of IL-17A, TNF-α, IL-10, and TGF-β in TCD8 cell population, compared to the mock and IVIG-treated conditions | |
| Zhu et al., 2017 [100] | Skin biopsies were taken from nonlesional and lesional skin from 5 AD patients | Upregulated KLK5 stimulated secretion of IL-8 and IL-10 With control of higher KLK5 activity by the inhibitor sunflower trypsin inhibitor G reduction in AD-related cytokine IL-8 and IL-10 secretion were observed. | |
| Malik et al., 2017 [101] | Biopsied lesions from 30 patients, 15 with positive house dust mite responses | HDM showed greater and more significant inflammation, including higher upregulation of markers related to general inflammation, IL-9, IL-22, and IL-19 vs. AD Both HDM and AD lesions showed significant upregulation of Th2 markers, some Th2 products were not as highly upregulated by HDM compared to chronic AD lesions IL-4, IL-10, IL-31, and IL-33, were not significantly upregulated in HDM APT responses vs. healthy skin, whereas the majority of these markers were significantly upregulated in AD lesions Th17-related markers (IL-8, IL-23p40) were strongly upregulated in HMD tissues showing greater similarity to nickel responses rather than to AD lesions, which showed lesser Th-17 skewing IL-22, and the IL-17/IL-22-induced S100As were significantly elevated in HDM tissues vs. controls, similar to AD lesions and nickel responses In AD patients, HDM showed greater immune activation across all inflammatory axes (IL-9, IL-22) IL-13, CCL22 and the Treg product FOXp3 showed more significant upregulation in healthy individuals Pathway enrichment analysis of the HDM transcriptome: • some were common to AD and psoriasis (T-cell receptor, NOD and toll-like receptor signaling, JAK-STAT signaling, interferon γ signaling), Th22 (IL-22) • specific to psoriasis (neutrophil, Th17 (IL-17, IL-12/23), tryptophan catabolism) • specific to AD [Th2 (IL-4), FCεRI signaling] | |
| Tan et al., 2017 [102] | Peripheral blood samples were collected from 87 children with AD and 60 healthy children as control subjects | The frequency of IL-17+ cells was higher in the dermis of patients with AD Serum IL-17 levels were higher in the AD group (10.47 ±3.39 pg/ml) compared with the control group (9.63 ±3.36 pg/ml), but this difference was not statistically significant |
Fairly consistent results can be found for IL-8 which was upregulated in AD derived mesenchymal stem cells when compared with healthy controls [79]. What is more, Yang et al. showed strong upregulation of IL-8 in AD skin lesions, which was inversely correlated with miR-124 [80]. Also, IL-10 despite being predominantly tested in the blood [81–83] appears to be reflected in biopsy specimens where its level is also upregulated [84], particularly in pruritic atopic skin lesions [85].
IL-13 mRNA expression level is elevated mainly in eczema lesions [86]. A high level of IL-13 was observed both in the lesional biopsy in adults [87] and in children [88]. Cell and biopsies studies also show that IL-13 mRNA level can be reduced by using steroids [85] or after ustekinumab treatment [89]. Although the results of blood cytokine levels vary in different studies, it can be confirmed that the level of IL-13 in blood collected from AD patients is higher when compared to psoriasis [90, 91]. Similar results were obtained for IL-19 [79, 86, 89] however, the level of IL-19 was significantly higher in children biopsies when compared to adults [88].
In the last few years, IL-17 is the most widely studied, especially subtype A. IL-17A was upregulated in AD-derived mesenchymal stem cells [79], TDP cells [92] as well as in biopsy lesions of an itchy AD [93] and conventional psoriasis [86]. Just like IL-19, IL-17A expression level was higher in children when compared to adults. However, Martel et al. was unable to detect the IL-17A or IL-17F in supernatants of cell cultures derived from either intrinsic or extrinsic AD [94].
Conclusions
In AD, the inside-out hypothesis has been favoured over the outside-in hypothesis, until mutations in genes encoding skin barrier proteins were discovered. According to the outside-in hypothesis, the skin barrier impairment, mainly linked to filaggrin mutations, is the main pathogenic factor in AD. However, most of the patients that have filaggrin mutations do not develop AD and only a quarter of the patients with AD have been diagnosed with a mutation in the filaggrin gene. It is clear now that the pathophysiological concepts underlying the development of AD are multi-factorial.
Atopic dermatitis research would strongly benefit from the unification of research methodology. General outlines systemizing material and methodology in AD research would contribute to the generation of high-quality data and publications that could be later analysed as a whole. This approach could accelerate the creation of new medical therapies and strategies.