An 8-month-old boy was admitted to the hospital with purpuric rash and oedematous ears appearing 1 day before his visit. The patient had a mild cough, nose discharge with no sputum, and fever preceding the onset of the rash. The rash began as oedema emerging from the auricles of both ears (Figures 1 A, B). On days 2–4 following the onset of the rash, lesions developed and gradually involved the patient’s cheeks, thighs, and limbs; they eventually became tender purplish-red plaques (Figures 1 C–H). On the fifth day following the onset of symptoms, the child’s oedema began to resolve and the lesions gradually faded (Figures 1 I–K). The lesions finally resolved with central crusting on the eighth day after symptoms first developed (Figure 1 I, L).
Figure 1
Lesions on the 1st and 2nd day of onset. On the 1st day: A – red, oedematous helix, B – red, oedematous helix and antihelix with few purpuric dots. On the 2nd day; C – ecchymotic purpura of the left cheek with a rosette-shaped border, D – targetoid, ecchymotic eruption of the right cheek. On the 3rd day: E – ecchymotic cockade purpura and oedema which extend to the mouth angle and angle of the jaw of the left face, F – the oedematous erythematous lesion extends to cover most of the helix and antihelix of the left auricle. On the 4th day: G – the targetoid, ecchymotic lesions confluent and extend to cover most of the right face, H – ecchymotic lesion extends more to cover most of the right face. On the 5th (I), 6th (J), and 7th (K) day of onset, lesions start to resolve by changing to brownish-red colour in figure. L – Lesions resolve leaving central crust on the 8th day of onset
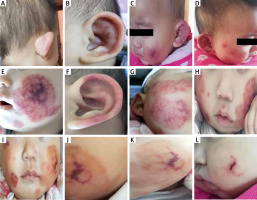
On physical examination, the child’s general condition was good; his pharynx was slightly red, and the heart and lung showed no auscultation abnormalities. Skin examination showed symmetrically distributed rosette-like purpuric petechiae and ecchymosis localized on both cheeks, ears, buttocks, and limbs with prominent oedema and tenderness. Some plaques showed a cockade pattern, and other plaques coalesced to form large purpuric lesions with polycyclic borders. Severe non-pitting oedema was noted on the patient’s face and limbs. No signs of mucosal or systemic involvement were found.
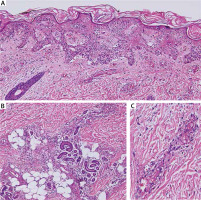
Laboratory examination at the time of admission revealed the following: white blood cells, 14.82 × 109/l (3.5–9.5 × 109/l); platelets, 621 × 109/l (125–350 × 109/l); red blood cells, 4.67 × 109/l (4–4.5 × 109/l); haemoglobin, 76 g/l (110–120 g/l); C-reactive protein, 36.20 mg/l (0–5 mg/l); D-dimer, 12.54 mg/l (0–0.5 mg/l); FDP, 36.36 µg/l (0–5 µg/l); ESR, 48 mm/h (0–15 mm/h); ferritin, 17.90 ng/ml (23.9–336.2 ng/ml); and vitamin B12, 165.81 ng/ml (180–914 pg/ml). Tests for liver function, myocardial enzymes, rheumatoid factor, autoantibodies, and urine analysis yielded normal findings. Ultrasound of the liver, gallbladder, pancreas, and spleen showed no abnormalities. Chest radiographs showed an increase in bilateral texture and cloudiness. Imaging revealed bronchial pneumonia. A biopsy of the lesions indicated features of leukocytoclastic vasculitis. At the time of admission, the following diagnoses were initially made: 1. skin infection, and 2. allergic purpura. Ceftriaxone and methylprednisolone were administered for 4 days and then discontinued after the diagnosis of acute haemorrhagic oedema of infancy (AHEI) was made based on the patient’s clinical and histopathological features (Figure 2). On the eighth day after admission, the patient’s lesions finally resolved as crusty patches on the face and he was discharged.
Figure 2
Histology shows orthokeratosis, spongiosis of the epidermis, lymphocytes extravasation is seen in the epidermis, dilated upper-dermis blood vessels with erythrocyte extravasation in all dermis levels (haematoxylin and eosin staining; original magnification 200×). (B) Deep perivascular infiltrate composed mostly of neutrophils with abundant nuclear dust; the inflammatory cells extend to the fat tissue; adnexal structures are unaffected. However, there is a mild inflammatory cell (haematoxylin and eosin staining; original magnification 200×). (C) higher magnification of (A) shows fibrinoid necrosis, neutrophilic nuclear dust (haematoxylin and eosin staining; original magnification 400×)
AHEI was first described by Snow in 1913 [1]. Since then, over 300 cases of this type of vasculitis have been reported in the literature [2]. AHEI mostly affects children aged below 2 years and is characterized by the concomitant occurrence of fever, purpuric plaques, and oedema [3, 4]. Painful oedema is also an associated feature of AHEI [5]. Rare associations between AHEI and kidney damage (as haematuria or mild proteinuria) and intestinal dysfunction (as bloody diarrhoea) have been reported [3]. No systemic involvement was observed in our present case. No specific laboratory test is yet available for AHEI. Although thrombocytosis is considered one of the inflammatory features of AHEI [6, 7], the exact aetiology of the condition remains unclear [6]. However, infections, especially of the upper respiratory and urinary tracts, drugs, and less commonly, immunization have been suggested to be factors triggering the condition [8]. In our case, leukocytosis, high CRP and ESR, and signs of bronchial pneumonia on X-ray were observed. Thus, infection may be the causative agent of the patient’s disease. The average interval between the possible trigger agent and the onset of AHEI ranges from 2 days to 30 days [9]. The dramatic onset of AHEI is worrisome for most parents, and assurance is often necessary, particularly because AHEI generally has a benign course. Purpuric lesions occurring in an infant must be examined via a thorough work-up to exclude more severe conditions, such as Henoch-Schönlein purpura, septicaemia, meningococcemia, Kawasaki disease, purpura fulminans, and erythema multiforme [10].
The treatment of AHEI remains controversial. Our patient was administered corticosteroids to hasten his recovery and avoid rapid progression of the disease. Thus, our patient’s lesions were cleared within 8 days. While steroids are not usually needed for lesion resolution, the use of short-term systemic corticosteroid therapy can help prevent the progression of the disease [6]. In our study, we documented the lesion resolution process by using a digital camera to show daily changes in lesion characteristics.








