Introduction
Psychodermatology is a relatively new field of medicine created from merging dermatology and psychiatry which explores complex interactions between the mind and the skin [1]. Occasionally, patients presenting to dermatologists may complain of symptoms primarily of psychiatric origin. Such is the case with individuals suffering from delusional infestation (DI) who have a false belief that their skin and body are infested by small vivid pathogens or inanimate objects [2]. DI has many synonyms in the literature, firstly described as acarophobie by Thibierge (1894), and was subsequently termed präseniler dermatozonewahn, delusions of parasitosis or Ekbom’s syndrome [3]. The annual incidence rate of DI was estimated as 16.6 cases per million inhabitants, with a prevalence of 83.21 cases per million. Women are approximately 2.5 times more often affected than men, although this observation concerns women aged 45 years and older suffering from primary DI [2]. Primary DI cannot be explained by other conditions and is a synonym of persistent delusional disorder (F22.0 according to the ICD-10 classification) or shared DI (induced psychotic disorder F24.0), whereas secondary DI occurs due to various conditions such as depression, schizophrenia, dementia, stroke, diabetes, HIV infection, medical drugs intake (e.g. ciprofloxacin), drug abuse (cocaine) or vitamin deficiencies [4–11], to mention just a few. The bizarre nature of DI is supported by the “specimen sign” (formerly described as the “matchbox sign” in 1983) [12]. It is considered a classic feature of DI and denotes samples of suspected parasites or other factors being brought for analysis by the patients. “Specimen sign” is a more appropriate term than “matchbox sign” as it focuses on any kind of samples being brought for examination, not the receptacle [2]. Due to its rarity, to date, most data on DI have come from case reports or small cases series.
Aim
This study aimed to analyse clinical features of a group of 21 subjects with DI who were admitted to a tertiary dermatology ward in order to aid clinicians in successfully performing their diagnostic and therapeutic procedures in this challenging group of patients.
Material and methods
A retrospective descriptive analysis of DI patients hospitalized between 1997 and 2019 in the dermatology department was carried out based on the available documentation. The diagnosis of DI was established upon typical clinical features and psychiatric consultation. The emphasis was put on the duration of symptoms, psychiatric symptomatology (including the presence of the specimen sign), systemic comorbidities, follow-up visit attendance as well as the initiated treatment and associated outcome.
Results
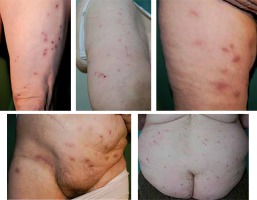
The data regarding 21 consecutive patients were obtained. The mean age of subjects was 65.2 ±13.3 years (range: 39–89 years), with the majority being females (n = 16; 76.2%). Regarding marital status, 9 were married (42.8%), 6 were widowed (28.6%), 3 were single and 3 were divorced (14.3% each). The majority of subjects (n = 15; 71.4%) reported living with at least one family member, 6 lived alone (28.6%). Fifty-seven percent of patients (n = 12) reported that they had visited a psychiatrist at least once before current admission. Among those, depression, anxiety disorder, alcohol dependence, benzodiazepine dependence and adaptive reaction had bothered one subject each. Coexisting comorbidities encompassed arterial hypertension (n = 6; 28.6%), mild anaemia (n = 3; 14.3%), hypothyreosis, type II diabetes and implanted cardiac defibrillator due to arrhythmia (n = 2; 9.5% each), rheumatoid arthritis, atopic dermatitis and psoriasis (n = 1; 4.8% each). Before being referred to the dermatology ward, 8 (38.1%) patients were treated for suspected scabies infestation with permethrin or crotamiton. The mean duration of symptoms prior to admission was 1.9 ±1.7 years (range: 1 month – 5 years). All patients presented with a variety of skin lesions attributed to chronic scratching or attempts to remove the imaginary parasites (erythema, erosions, excoriations, crusts, hyperpigmentation, scars), usually located on the trunk and lower limbs (Figure 1). The specimen sign was present in 47.6% of cases (n = 10), usually consisting of dirt, sand, threads or debris presented in a plastic container (Figure 2). Among those, 3 patients also drew the images of the suspected causative factors on a piece of paper. Patients provided abundant verbal descriptions of suspected causative factors of their symptoms (Table 1), with worms (n = 11; 52.4%), unspecified parasites (n = 9; 42.9%), “something” (n = 7; 33.3%) and flies (n = 4; 19%) reported most commonly. The need of a psychiatric consultation by a dedicated psychiatrist was explained to all patients and each agreed to participate. Overall, a psychiatric consultation was performed in 20 (95.2%) patients (1 patient left the ward without informing medical personnel of his intentions before being consulted). Primary delusional disorder was diagnosed in 16 (76.2%) patients (F22.0), followed by shared delusional disorder (F24) and secondary delusional disorder of organic origin (2 patients each; 9.5%) (F06.2). Regarding the diagnosis of shared delusional disorder (folie a deux), 2 patients (a 79-year-old male and a 66-year-old female) were a married couple. The belief of infestation had started 5 years earlier in the woman. They expressed that the “worms” appeared because of the homeless person who periodically resided in the garden shed. The couple consulted dermatologists, parasitologists, veterinarians and entomologists. They applied household detergents to their skin which resulted in skin irritation. A disinfection of the apartment was performed thrice; the furniture was also removed.
Table 1
Sociodemographic and clinical data of psoriasis patients (with and without depression) and healthy controls. Median value: 25–75%
Risperidone was initiated in 61.9% of patients (n = 13), usually starting with 0.5–2 mg nightly, with a gradual dose increase. Among patients treated with risperidone, haloperidol monotherapy preceded treatment with risperidone in 1 patient, whereas unsuccessful olanzapine course was followed by instigating risperidone in the other patient. Other treatment modalities included perazine, promazine, haloperidol (in monotherapy or in combination with paroxetine) and chlorprothixene. Two (9.5%) patients refused to initiate treatment with risperidone. Unfortunately, only 7 patients (33.3% in total) attended the follow-up visit, whereas a partial remission of symptoms was observed in 5 of them (23.8% in total). One patient decided to discontinue risperidone due to adverse events (dizziness, tachycardia), while the other one due to no improvement of the symptoms perceived.
The summary of clinical data regarding the subjects is presented in Table 2.
Table 2
Correlations between subscales of TEMPS-A in psoriasis patients (Spearman’s rho)
Discussion
To the best of our knowledge, this is the first report on DI among Polish patients involving such a big series of patients. DI is typically considered as a rare condition; nevertheless, our previous study suggests that it is commonly encountered in dermatological practice [13]. Approximately 85% of 118 dermatologists responding to the survey reported that they had seen at least 1 patient with DI during their practice. Frequently, the phenomenon of “doctor hopping” occurs. Patients attend various dermatologists, tropical disease specialists, as well as entomologists and microbiologists [2]. As reported in the present study, general practitioners and dermatologists often initiate topical treatment with anti-scabietic drugs. Occasionally, such treatment is instigated together with antipsychotics in order to gain patient’s trust. Usually, establishing the diagnosis does not pose any difficulties due to the characteristic clinical picture. The specimen sign mostly involves dirt particles, sand, debris, fragments of epidermis, hair or cloth threads stored in plastic jars or bags; drawings, photographs or videos may also be presented. It is a classical yet not mandatory sign of DI; its prevalence in our study (10 out of 21 patients; 47.6%) was similar to other reports [14, 15].
Treatment of DI is problematic as patients may refuse to take psychiatric drugs and do not attend the follow-up visits. To establish trustful rapport it is necessary to carefully examine the specimens brought by the patients [16]. This also includes dermoscopy [17]. Additionally, the physician might consider taking a biopsy if the patient expects it [18]. The latter solution is based on two conditions firmly explained to the patient: the biopsy site is chosen by the physician and the patient agrees to reconsider his/her beliefs if the results of histological examination do not support the presence of parasites. Tactile hallucinations, paraesthesias and pruritus are frequently experienced by the patients, yet paradoxically they may be considered physician’s “allies” as the instigation of “psychiatric” drugs relieves these bothersome symptoms. Thereby, the expectations of both the patient and the physician are met, despite critical differences in the views concerning aetiology of the symptoms. In extremely rare cases though, the symptoms in subjects initially diagnosed with DI would eventually turn out to be truly associated with parasites, e.g. with Limothrips cerealium (grain thrips) or Dryomyza formosa (intestinal myiasis) [19, 20].
Antipsychotics constitute the main therapeutic modality of DI, although in most cases it is difficult to convince patients to initiate such treatment. In our patients psychiatric consultation was performed in the presence of the dermatologist, which might have contributed to higher initial compliance to treatment. A lack of willingness to attend the psychiatric consultation and refusal to instigate or continue psychoactive medication stem from the lack of insight and criticism of the affected patients. These aspects are direct consequences of psychotic symptomatology, such as delusions and tactile sensations. A first-generation antipsychotic, pimozide (currently unavailable in Poland), has been studied in the literature featuring the largest number of DI patients so far [14, 21, 22]. The newer substances belonging to the second-generation (atypical) antipsychotics also act as dopamine receptor (D2) antagonists; however, their antagonism towards serotonin receptors (particularly the 5-HT2a) is more pronounced. Therefore, they still exert a high antipsychotic effect whilst reducing the risk of adverse events, such as the extrapyramidal syndrome [23, 24]. Currently, risperidone is considered as the drug of choice in DI [25]. The starting dose is usually 0.5–2 mg nightly, gradually increased to 4–8 mg/day. Notably, risperidone doses utilized in DI are usually smaller than in schizophrenia. A Japanese case report demonstrated a significant increase in regional cerebral blood flows in the bilateral frontal and left temporoparietal regions, the right parietal operculum and the bilateral basal ganglia after initiating risperidone in a patient with DI [26]. Compared to first-generation antipsychotics, risperidone is associated with a higher risk of metabolic syndrome, hyperprolactinemia and postural hypotension [24]. Moreover, an association with cerebrovascular incidents has also been observed in several studies among the elderly [27–29], although this association is less pronounced than in patients treated with haloperidol or chlorpromazine [30] and similar to those using olanzapine or quetiapine [31]. Nevertheless, this drug should not be used in patients who underwent a stroke recently. Other atypical antipsychotics mentioned in case reports encompass aripiprazole (3.75–15 mg/day), olanzapine (2.5–10 mg/day), paliperidone (3 mg/day), quetiapine (25–50 mg/day), sertindole (4–12 mg/day) and ziprasidone (120 mg/day) [32–39]. Unfortunately, long-term treatment with antipsychotics lasting from months or years is often necessary to maintain remission as early discontinuation of the drug following the initial success causes recurrence of symptoms.
Early instigation of treatment is relevant as the patients’ symptoms may intensify, resulting in a suicidal attempt and occasionally leading to death [40]. During the clinical interview DI patients often appear reluctant to cooperate and secretive while verbal and physical aggression towards medical personnel also occurs occasionally. Remarkably, there was a report of an elderly female with DI who attempted to shoot her doctor with a hunting gun (fortunately she missed) [41]. The paper also mentions a personal communication referencing an unpublished case of a female with DI who shot her physician after being suggested to visit a psychiatrist.
Conclusions
DI patients present mainly to dermatologists despite the psychiatric origins of the disease and feature a variety of bizarre clinical manifestations. Based on the literature and similarly to other delusional disorders, DI may be treated with antipsychotics, both first-generation and atypical. Currently, risperidone is considered the mainstay of treatment. Managing these patients remains a challenge and requires strict cooperation between dermatologists and psychiatrists.