Introduction
Due to the rising incidence of cutaneous melanoma in Europe and increasing social awareness, the number of patients who report to dermatologists in order to assess their skin changes is growing rapidly [1, 2]. For examining melanocytic skin lesions, dermoscopes have currently become the first-line diagnostic tools since they allow the in vivo examination of structures in the epidermis and superficial dermis which are not accessible for naked-eye examination [3–5].
The first detailed description of the microscopic assessment of the surface of the skin was presented by Saphier, who also introduced the term dermatoscopy for the first time in the 1920s. It was not until 1951 in the USA that Goldman first described the dermoscopic image of melanocytic nevi. The rapid development of dermoscopy as a method of assessing melanocytic changes in the skin occurred in the 1980s, mainly due to the work of Soyer and Pehamberger [6]. The next step was the introduction of videodermoscopy as a technique for recording and comparing images of selected skin lesions, enabling dermatologists to diagnose early stages of cutaneous melanoma [7].
In modern dermatological practice, dermoscopy (epiluminescent microscopy) is considered a non-invasive, easily reproducible and inexpensive method of the in vivo assessment of structures and colors within the epidermis and superficial dermis. It allows examination using 10–20× magnification – in traditional handheld dermoscopes – and 10–200×magnification in videodermoscopes [8]. Dermoscopy enables the clinician to examine plural diagnostic features in different skin lesions, which are normally impossible to detect with the naked eye. As a vastly researched technique it is known to have a high specificity of ≈ 65–90% and sensitivity evaluated as ≈ 65–90% [9–11] if performed by a well-experienced clinician [7, 12].
Breslow’s depth – described in the 1970s – is a method that allows the pathologist to assess the stage of melanoma by measuring the depth of infiltration in millimeters [13]. At first staging the cutoff was applied for Breslow’s depth of 0.76 mm, later updated by the American Joint Committee on Cancer to 1.0 mm (or < 1.0 mm when ulceration is present) [13, 14].
Advanced metastatic melanoma is still considered incurable in most cases, whereas early cutaneous melanomas are curable by means of surgical excision. According to guidelines in patients with cutaneous melanomas stage ≤ pT1a, surgical excision within the range 0.5–1 cm [15, 16] is considered curative. In patients with > pT1a melanomas, usually sentinel lymph node biopsy and often subsequent surgery remain unavoidable [15–17]. Even though there are plural emerging therapies introduced in the treatment of metastatic melanomas, it still remains the case that the more advanced the disease the worse the prognosis (overall survival) for the patients [18–20]. This is why an early, accurate diagnosis is crucial for positive outcomes. Nowadays, despite the emergence of diagnostic tools such as reflectance confocal microscopy [21–24], and optical coherence tomography [25], which have been described as being more specific and sensitive than dermoscopy [24, 26, 27], their accessibility is still limited, and dermoscopy remains a first-line diagnostic tool for dermatologists [25, 28].
Aim
In the study, the goal was to decide whether there is a significant difference between the presence or absence of each of the dermoscopic features listed in Table 1 [10, 29, 30], compared to the location on the skin and pathology results: Breslow’s depth, mitotic index and ulceration.
Table 1
Material and methods
In our study, we retrospectively evaluated videodermoscopic images of histopathologically confirmed melanomas in 81 patients that were diagnosed and examined at the Dermatology Department, Jagiellonian University Medical College in Krakow, Poland. The dermoscopic images were acquired using videodermoscopes (Fotofinder, TeachScreen GmbH, Bad Birnbach, Germany). For every lesion a set of at least 3 images was acquired. Entire lesions were photographed using 20× magnification. In addition, local features of tumors were photographed using maximum 120× magnification. The image data were collected between 2013 and 2017. The evaluation was conducted by two dermatologists with a minimum of 6 years’ experience in general dermatology and dermoscopy. For the purpose of blinding the images were anonymized. For all the evaluated tumors, the patients’ data were collected, including: age (at the time of diagnosis), sex, location of the primary tumor, Breslow’s depth, the histological subtype of melanoma, mitotic index and information on the presence/absence of ulceration.
The examining dermatologists evaluated the lesions assessing the presence or absence of the following dermoscopic features: white regression structures; peppering; blue-white veil; atypical blood vessels/atypical vascular structures; atypical pigment network; atypical dots and globules; irregular streaks; a multicomponent (complex) pattern; pseudopods and nodules. The definitions of listed structures are available in Table 1.
In the study, we evaluated the association between the presence of the dermoscopic structures (listed in Table 1) and histopathological stage of melanoma – by dividing tumors into: in situ, thin invasive tumors (using cutoff at ≤ 1.0 mm) and thick invasive tumors (cutoff at > 1.0 mm). We also assessed the predictors in accordance with the mitotic index (0 or ≥ 1) and presence of ulceration.
Dermoscopic images of acral, nail apparatus, spitzoid and amelanotic melanomas were excluded due to differences in the dermoscopic picture of such tumors.
Results
The group of patients consisted of 44 men and 37 women aged (at the day of diagnosis) between 26 and 97 with the average being 61.4 and the median being 63.
Regarding the distribution of the lesions, 14 (17.28%) were found on the head, 37 (45.68%) on the trunk, 16 (19.75%) on the upper limbs and 14 (17.28%) on the lower limbs.
Twenty-two (27.16%) of 81 melanomas were in situ, and 59 (72.84%) were invasive. On pathology examination in 6 (7.5%) tumors ulceration was detected and in 23 (28.4%) lesions the mitotic index was ≥ 1.
Comparison of the prevalence of the listed dermoscopic features (Table 1) between male and female patients showed a statistically significantly higher prevalence of atypical blood vessels and a multicomponent pattern in female patients (62.2 and 73% respectively; p < 0.005).
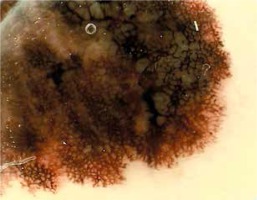
Assessing the relation between evaluated dermoscopic structures and different locations of primary tumors (head, trunk, lower limbs, and upper limbs) (Table 2) we found that pseudopods were most commonly present on the lower limbs (50%), less commonly on the trunk and upper limbs (24.32% and 25% respectively; p = 0.024). An atypical network was described in 66.67% of all tumors, ranging between 78.57% and 78.38% on the lower limbs and trunk, and 42.86% and 50% on the head and upper limbs (Figure 1).
Table 2
Frequency of various dermoscopic features depending on the location on the skin (head, trunk, upper and lower limbs)
In situ melanomas showed a significantly lower frequency of pseudopods and multicomponent pattern (9.1% and 36.4%) in comparison with invasive tumors – with 30.5% and 69.5% positive for pseudopods and multicomponent pattern (Table 3).
Table 3
Frequency of various dermoscopic features of in situ and invasive melanomas
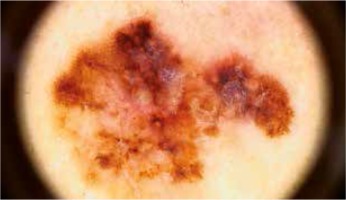
Tumors thicker than 1.0 mm showed a higher prevalence of white regression structures with 22.7% sensitivity, though 100% specificity. An atypical network was observed in 46.7% of tumors > 1.0 mm thick and in 71.2% of thinner tumors, though the difference was not statistically significant (Figure 2). In 60% of melanomas > 1.0 mm thick and 21.2% of melanomas ≤ 1.0 mm nodules were described in dermoscopy.
Figure 2
Atypical network (blue dots), white regression structures and atypical blood vessels (red dots) in invasive melanoma of the trunk
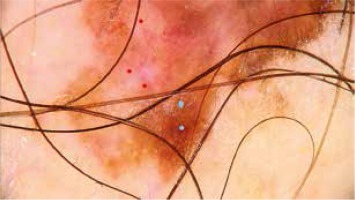
The study also showed a negative correlation between the positive mitotic index and an atypical network, and positive correlation between MI ≥ 1 and nodules as well as a positive correlation for presence of ulceration and atypical blood vessels/vascular structures and nodules on dermoscopy.
Discussion
The last three decades have brought rapid development of diagnostic tools in dermato-oncology. Due to their accessibility, dermoscopy and videodermoscopy are the most commonly used diagnostic techniques in the early diagnosis of cutaneous melanoma [8, 31]. In order to standardize dermoscopic examinations specific diagnostic features, as well as criteria, have been described. The most popular algorithms applied in the dermoscopic diagnosis of melanoma are: pattern analysis [6], ABCD criteria [9, 32, 33], and the 7-point checklist [3, 34]. Knowing the listed criteria allows the early detection of cutaneous melanomas by an experienced dermoscopist in contrast to naked-eye examination. It would be extremely useful if it could also help clinicians to determine the tumor stage or Breslow’s depth, in order to simplify the planning of excisions and possible sentinel lymph node biopsies.
In previous studies, there have been a few attempts to examine the possible correlations between the presence of distinct dermoscopic features and tumor thickness, though the results are contradictory.
In a study on 123 melanomas (including only tumors with a Breslow’s depth of > 0.75), González-Álvarez et al. observed a correlation of dermoscopic ulcerations and blotches with positive sentinel lymph node biopsy results and the presence of an atypical pigment network with negative sentinel lymph node biopsy results [35]. Pizzichetta et al. examined and compared dermoscopic images of in situ melanomas and invasive melanomas (subdivided into two groups ≤ 0.75 mm and > 0.75 mm), finding a lower prevalence of atypical pigment network and pseudopods in thick invasive melanomas (> 0.75 mm) in comparison with in situ melanomas, as well as greater relative numbers of pseudopods, brown globules, gray-blue areas, and depigmentation in in situ lesions compared with invasive melanomas with a Breslow’s depth of ≤ 0.75 mm. On the other hand, Menzies et al., researching the morphologic criteria of pseudopods in 80 melanomas (62 invasive and 18 in situ) and 159 randomly selected pigmented nonmelanomas, observed higher prevalence of the structures in invasive melanomas in comparison to in situ tumors. Pseudopods retained 97% specificity, though only 23% sensitivity [36]. Emiroglu et al., researching only trunk melanomas (71 cases), found no correlation between dermoscopic image and Breslow’s depth [37].
In our study using univariate regression analysis, we compared the prevalence of the listed dermoscopic features (Table 1) between male and female patients, and found a significantly higher prevalence of atypical blood vessels and a multicomponent pattern in female patients (62.2% and 73% respectively; p < 0.005). The analysis showed no significant differences in different age groups.
The χ2 analysis was performed to assess the relation between evaluated dermoscopic structures and different locations of primary tumors (head, trunk, lower limbs, and upper limbs) (Table 2). There were no significant differences in the presence of regression, peppering, atypical blood vessels, atypical dots and globules, multicomponent pattern or nodules. Pseudopods were present in 24.69% of all evaluated tumors; most commonly they were observed on the lower limbs (50%), less commonly on the trunk and upper limbs (24.32% and 25% respectively; p = 0.024). An atypical network was described in 66.67% of all tumors, ranging between 78.57% and 78.38% on the lower limbs and trunk, and 42.86% and 50% on the head and upper limbs.
In order to analyze possible relationships between the presence of the listed dermoscopic features and histopathologic stage of melanomas, the Mann-Whitey U test and Student’s t test were used. In situ melanomas were evaluated separately as well as tumors presenting Breslow’s depth ≤ 1 mm and > 1 mm to check for differences in frequency of appearance of distinct dermoscopic structures. No statistically significant differences regarding the prevalence of peppering, blue-white veil, atypical dots and globules, atypical blood vessels or streaks were noted (Table 4). In situ melanomas showed a significantly lower frequency of pseudopods and multicomponent pattern (9.1% and 36.4%) in comparison with invasive tumors (Figure 3) – with 30.5% and 69.5% positive for pseudopods and multicomponent pattern (Table 3).
Table 4
Frequency of dermoscopic features in melanomas ≤ 1.0 mm and > 1.0 mm on Breslow’s depth
We have calculated mean Breslow’s depth in melanomas divided into groups depending on the presence/absence of dermoscopic features. Tumors with a visible atypical pigment network had a significantly lower mean Breslow’s depth in comparison to melanomas presenting no atypical pigment network. Melanomas presenting with nodules, on the other hand, had greater Breslow’s depth in comparison to tumors presenting no nodules on dermoscopy (Table 5).
Table 5
Mean Breslow’s depth in melanomas divided into groups depending on the presence/absence of dermoscopic features
Taking into account the guidelines updated by the American Joint Committee on Cancer (as well as many European, including Polish), we compared thick and thin melanomas assuming a cutoff point at 1.0 mm Breslow’s depth. Tumors thicker than 1.0 mm showed a higher prevalence of white regression structures with 22.7% sensitivity, though 100% specificity. An atypical network was observed in 46.7% of tumors > 1.0 mm thick and in 71.2% of thinner tumors, though the difference was not statistically significant. In 60% of melanomas > 1.0 mm thick and 21.2% of melanomas ≤ 1.0 mm nodules were described in dermoscopy. Specificity of this feature was 89.7% in thick melanomas and sensitivity 39.1% (p = 0.003; OR = 5.57).
Considering the fact that the mitotic index was removed from AJCC guidelines in 2018, and replaced with ulceration, but is still applied in melanoma staging and grading in many European countries [14, 15, 38], we analyzed the presence of dermoscopic structures in melanomas with a negative mitotic index in comparison to melanomas with MI ≥ 1. The Mann-Whitey U test showed a negative correlation between the positive mitotic index and an atypical network, and a positive correlation between MI ≥ 1 and nodules.
Controlling for ulceration in pathology, we found a positive correlation for atypical blood vessels/vascular structures and nodules with low sensitivity (13.9% and 18.2% respectively; p < 0.05) but high specificity.
Conclusions
The presence of an atypical network may point the examining dermatologist towards the diagnosis of thin invasive melanoma, whereas pseudopods, a multicomponent pattern, white regression structures, atypical blood vessels, and nodules suggest invasive (high stage) melanoma. Thus the presence of such dermoscopic structures should alert the examining dermatologist. Dermoscopy is a very useful method in the early diagnosis of cutaneous melanoma, especially in regard to early, non- metastatic tumors. It may also provide some insight into the metastatic potential of detected tumors.