Introduction
Liver biopsy (LB) helps diagnose diffuse liver disorders and focal liver lesions. The conventional method of obtaining biopsy samples is through a percutaneous route under ultrasound guidance [1]. Transjugular or plugged liver biopsy is performed in conditions such as ascites and coagulopathy where percutaneous sampling is contraindicated [2, 3]. With the increasing use of endoscopic ultrasonography (EUS) in clinical practice, EUS is being used to sample liver tissue as it offers a few advantages compared to conventional modes of tissue acquisition.
As the trajectory of the needle through the course of the liver can be viewed in real time, intrahepatic vessels and the bile duct can be avoided [4]. EUS allows access to both lobes of the liver, and the fanning technique helps acquire representative samples and multiple cores. Patient discomfort is minimal as they are adequately sedated throughout the procedure. EUS helps in the identification of smaller lesions in the liver and aids in the evaluation of surrounding abdominal structures. Liver lesions identified while staging upper abdominal malignancies can also be biopsied in the same setting without requiring an additional procedure. EUS-LB is particularly helpful in liver lesions not accessible by ultrasound (US) or computed tomography (CT) [5]. It also has a particular advantage in obese individuals requiring liver biopsy [4].
A meta-analysis reported a histologic diagnosis rate of 93.9%, with a 2.3% incidence of adverse events with EUS-LB [6]. EUS-LB was comparable to percutaneous liver biopsy (PLB) in terms of the total specimen length (TSL) and complete portal tracts (CPT) obtained, with no difference in the incidence of severe adverse events [7]. Despite its advantages, EUS-LB has not been widely adopted in clinical practice due to concerns regarding tissue fragmentation, tissue sufficiency, and bleeding risk. Presently, data on EUS-LB from India are scarce. Hence, we aimed to study the safety, efficacy, and histological adequacy of EUS-guided liver biopsy and its predictors.
Material and methods
This is a retrospective analysis of prospectively maintained data from January 2021 to October 2022 of consecutive patients undergoing EUS-LB at four tertiary care centers in India. This study has been carried out in accordance with the Declaration of Helsinki after the approval of respective ethical committees.
Patient selection
Study participants were adults (≥ 18 years) with a need for liver biopsy as per consultation with a gastroenterologist for evaluation of the cause of deranged liver function tests (LFT), jaundice, or portal hypertension. Contraindications for EUS-LB included using antiplatelets or anticoagulants within the last five days, platelet count < 50 000/µl and international normalized ratio (INR) > 1.6, inability to provide informed consent, moderate to gross ascites, and pregnancy.
Techniques
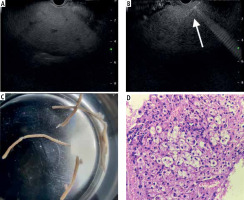
All procedures were performed with the patient in a left lateral position, under sedation with propofol or a combination of midazolam and pentazocine with continuous cardiorespiratory monitoring by an experienced endoscopist (> 300 EUS procedures). A standard linear-array echoendoscope (GF-UCT180, Olympus) was utilized to evaluate the visualized liver and rule out tumors or extrahepatic causes of deranged liver function tests. The transgastric route was used for the left lobe (Fig. 1A), and the transduodenal route for the right lobe access. All EUS-LB were performed using a 19-G or 22-G FNA (Echotip Ultra, Cook Medical, Bloomington, Ind, USA) or Franseen FNB needle (Acquire, Boston Scientific Co., Ltd., Natick, MA, USA), depending on the availability at the center. One to three passes were performed under real-time view (Fig. 1B) depending on the macroscopic on-site evaluation. The choice of suction techniques was at the discretion of the endoscopist. For the slow stylet suction method, the stylet was gradually withdrawn during actuation after the initial pass. The needle was primed with heparin for wet suction. A syringe filled with 10 ml of saline with a vacuum suction of 10 ml was attached to the needle. After the puncture into the liver, the vacuum suction was released while making actuations into the liver. Core biopsy specimens were then transferred to a slide to assess the total length of the core and the longest segment was retrieved and then placed into 10% formalin to send for pathological evaluation. A macroscopic assessment of core tissue was performed (Fig. 1C). After the procedure, patients were observed for at least 4 hours with periodic vital monitoring. Gastrointestinal pathologists at respective centers with experience of more than ten years examined the tissue blocks after staining with hematoxylin and eosin (Fig. 1D). Special stains (e.g., CK7) were used as and when required.
Fig. 1
A) Identification of the left lobe of the liver from the transgastric route; B) Puncture done with biopsy needle under real-time view; C) Macroscopic inspection of the sample for adequacy; D) Hematoxylin and eosin staining showing ballooning degeneration in a case of non-alcoholic steatohepatitis
Study outcomes
The study’s primary outcome was sample adequacy based on TSL and the number of CPTs. A CPT is defined by the presence of all three portal structures within a complete circumference, including branches of the portal vein, hepatic artery, and bile duct. There is variation concerning the definition of sample adequacy for liver biopsy specimens. A TSL of ≥ 15 mm with ≥ 6 CPTs is usually considered adequate as per the EASL criteria, while the AASLD criteria define adequacy as a TSL of ≥ 20 mm with ≥ 11 CPTs [8]. For the present study, a sample with TSL of ≥ 15 mm and at least 6 portal tracts were considered adequate. A sample with TSL of ≥ 20 mm and 11 or more CPTs was considered as optimal. The secondary outcomes of the study were the rate of successful pathological diagnoses and the rate of adverse events (AE). We also aimed to analyze the factors associated with the acquisition of an optimal sample.
Statistical analysis
Continuous data were expressed as median and range or mean and standard deviation based on the test of normality. Categorical data were expressed as frequency and percentage. Dichotomous variables were compared using the chi-square (χ2) test or Fisher’s exact test. Parametric or non-parametric tests were used for comparing continuous variables. Multivariate analysis using a logistic regression model was used to identify independent predictors of an optimal sample. A p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software version 20.0.
Results
Baseline characteristics
A total of 74 patients (median age: 44.5 years, 50.0% males) who met the inclusion criteria were recruited. Table 1 outlines the baseline characteristics of the study population. Ascites was present in 11 (14.9% of cases) while 14 (18.9%) patients had associated esophageal varices. The common indications were abnormal LFT without jaundice (32, 43.2%), jaundice (21, 28.4%), evaluation of chronic liver disease (CLD) or portal hypertension (15, 20.3%), and suspected infiltrative liver disease (6, 8.1%) (Table 2).
Table 1
Baseline characteristics of the patients undergoing endoscopic ultrasound-guided liver biopsy
Table 2
Indications for liver biopsy and final diagnosis
Procedure details and adverse events
Table 3 summarizes the procedural details of the present study. The majority of the patients underwent left-lobe biopsy (62/74, 83.8%), while 1 (1.3%) underwent only a right-lobe biopsy, and 12 (16.2%) underwent a bilobar biopsy. A 19-G Franseen FNB needle was most commonly used (61/74, 82.4%). The median number of needle passes was 1 (range: 1-3) with a median of 5 (range: 2-12) actuations. The median number of actuations in patients with ascites was 3 (range: 2-8) compared to 5 (range 2-12) in those without (p = NS). Concerning the suction type, wet heparin suction was used in most cases (60/74, 81.1%), while slow stylet pull was used in 14 (18.9%) cases. There were five mild AEs (6.8%) observed. One patient had mild self-limited oozing from the puncture site. Four others complained of post-procedural pain responding to analgesics. There were no sedation-related AEs.
Table 3
Procedural details of the endoscopic ultrasound-guided liver biopsy and adverse events
Histopathological examination
The histopathological outcomes are summarized in Table 4, with the comparison of unilobar and bilobar biopsy reported in Table 5. The median longest single specimen was 25 mm (range: 6-46) with a median TSL of 60 mm (range: 11-140). The median number of CPTs was 12 (range: 3-34). Adequate and optimal samples were seen in 71 (95.9%) and 49 (66.2%) cases. A conclusive diagnosis was achieved in 97.3% (72/74) of the patients. The commonest diagnosis was non-alcoholic steatohepatitis (24, 32.4%), followed by autoimmune hepatitis (10, 13.5%), drug-induced liver injury (10, 13.5%), and cholestatic liver disease (7, 9.4%) (Table 2).
Table 4
Histopathological outcomes of endoscopic ultrasound-guided liver biopsy
Table 5
Comparison of histopathological outcomes between unilobar vs. bilobar biopsy
Predictors of optimal sample
We compared the parameters between those with and those without an optimal sample (Table 6). There was a significant difference between the groups regarding the presence of ascites and the site of biopsy (single or bilobar). A multivariable analysis was performed to assess the predictors of an optimal sample using the AASLD criteria. The presence of ascites was associated with a suboptimal sample (odds ratio [OR] = 0.128, 95% CI: 0.017-0.96, p = 0.046) (Table 5).
Table 6
Comparison of parameters between patients with or without optimal sample as defined by AASLD criteria
Discussion
The acquisition of liver tissue remains an essential step in diagnosing, staging, and treating various benign and malignant liver disorders. In recent years, EUS-LB has become an increasingly popular method of tissue acquisition for evaluating both focal and diffuse liver disease. However, concerns regarding tissue quality prevent its widespread use. The present study reported an adequate sample in 95.9% of cases and minor AE in 6.8% of cases. Using AASLD criteria, 66.2% of patients had an optimal sample. A final diagnosis could be obtained in 97.3% (72/74) of cases. On multivariate analysis, the presence of ascites was the only negative predictor for optimal sample acquisition.
A previous meta-analysis of 33 studies on EUS-LB reported a 95% pooled rate of diagnostic yield and 84% specimen adequacy with a 3% incidence of AE [9]. On subgroup analysis, the diagnostic yield with Franseen needles was higher than with fork-tip needles (99% vs. 88%, p = 0.047), and the rate of AEs was higher with FNB needles compared to FNA needles (6% vs. 1%, p = 0.028). Aggarwal et al. prospectively compared the outcome of EUS-LB of the left lobe sequentially using 19-G fork-tip and Franseen FNB needles [10]. Failure to achieve a final diagnosis was significantly higher with a fork-tip FNB system (20.6% vs. 2.8%) with a lower mean number of CPTs and specimen length. The study reported that at least 2 needle passes with 19-G FNB are required to obtain an adequate sample as defined by the AASLD criteria. In the present study, the number of passes varied from 1 to 3, with actuations varying from 2 to 12. This may have been due to variations in the needle type and size.
With a percutaneous biopsy, the right lobe of the liver is usually targeted. A recent study from India compared left-lobe EUS-LB with right-lobe and bilobar biopsy [11]. The mean cumulative length and CPT were comparable between the left and right lobes. Diagnosis between the two lobes showed substantial concordance with bilobar biopsies. In the study by Diehl et al., there was no significant difference in the aggregate tissue length and the number of CPTs yielded by left, right or bilobar biopsy [12]. In the present study, 85.1% of samples were obtained from a single lobe, with a similar diagnostic outcome as bilobar biopsy (final diagnosis achieved in 96.8% vs. 100%, p = 1.000). Thus, EUS-LB from the left lobe provides an adequate sample with similar diagnostic accuracy as bilobar sampling.
Shah et al. analyzed the diagnostic outcome of EUS-LB, comparing 19-G and 22-G core needles using a single-pass, single-actuation, wet suction technique [13]. The cumulative core length (2.5 cm vs. 1.2 cm, p < 0.0001) and number of CPTs (5.8 vs. 1.7, p < 0.0001) were significantly higher with the 19-G needle than the 22-G needle. Both sample adequacy (as per AASLD criteria) (60% vs. 5%, p < 0.001) and diagnostic sample rate (85% vs. 10%, p < 0.001) were higher with the 19-G needle than the 22-G needle. In the present study also, 94.6% of patients underwent EUS-LB with a 19-G needle with an optimal sample in 66.2% of cases, comparable to the previous study.
Concerning the optimal number of passes required for a diagnostic sample, Ching-Companioni et al. compared the diagnostic outcome of single versus multiple needle actuations for EUS-LB [14]. Specimens obtained using three actuations had significantly higher CPTs (17.25 ±6.2 vs. 24.5 ±9.88, p < 0.008) and a longer aggregate specimen length (6.89 ±1.86 cm vs. 12.85 ±4.02 cm, p < 0.001) compared to 1 actuation. In the present study, the median number of actuations was lower in patients with ascites (3 vs. 5), but the difference was non-significant. Hence, it is possible that in patients with ascites, the number of actuations would have been lower, leading to a suboptimal sample in the present study.
Priming the needle with heparin reduces the specimen’s bloodiness without interfering with the histopathology results [15]. In a prospective cross-over study comparing wet and dry suction for EUS-LB, specimen adequacy (aggregate specimen length ≥ 15 mm and ≥ 5 CPTs) was higher with wet heparin than with dry suction (98% vs. 80%) [16]. In the present study also, wet suction was used in 81.1% of patients, leading to sample adequacy in 95.9% using the same criteria. Hence, the evidence suggests wet suction’s superiority over other suction techniques.
In a previous randomized trial, EUS-LB was done with a 19-G FNB needle without suction or fanning, with two passes (10 actuations/pass) per patient [17]. EUS-LB was associated with a lower proportion of optimal specimens (defined as the length of > 25 mm after fixation and presence of ≥ 11 CPT) (57.9% vs. 23.8%, p = 0.028) and smaller median sample length (26 mm vs. 16.5 mm, p = 0.004) compared to PC-LB. Despite this, a final diagnosis could be made in all the samples obtained with either of the methods. The post-procedure visual analog scale (VAS) score for pain was significantly lower for EUS-LB at 1 hour, but there was no difference in the rate of adverse events. The authors concluded that PC-LB was better than EUS-LB in terms of sample adequacy. However, the criterion (> 25 mm length) used for defining adequacy is not defined in any of the current guidelines. Also, the authors used a no-suction technique, which may have led to a lower yield.
A recent study from Italy reported a comparable diagnostic accuracy between the EUS-LB group and the PC-LB group (88.8% vs. 100%, p = 0.82) [18]. In a recent meta-analysis comparing EUS-LB with PC-LB, pooled specimen length was comparable between EUS-LB (29.9 mm, 95% CI: 24.1-35.7) and PC-LB (29.7 mm, 95% CI: 27.1-32.2) with a mean difference of –0.35 mm (95% CI: –5.31-4.61, p = 0.89) [7]. Moreover, the pooled number of CPTs (12.9, 95% CI: 7.7-18 with EUS-LB vs. 14.4, 95% CI: 10.7-18 with PC-LB) and severe AE (OR = 1.11, 95% CI: 0.11-11.03) were comparable between the two groups. Hence, the diagnostic performance and safety profiles of EUS-LB and PC-LB are comparable. Table 8 summarizes the advantages and disadvantages of EUS-LB and PC-LB.
Table 7
Multivariate analysis of predictors of optimal sample with endoscopic ultrasound-guided liver biopsy
Table 8
Comparison of advantages and disadvantages of endoscopic ultrasound-guided liver biopsy with percutaneous liver biopsy
To our knowledge, this is the largest study on the diagnostic outcome of EUS-LB from India. Multicentric data add to the strength of the study. Despite this, there are several limitations to the present study, the retrospective nature being the first. There is no comparator arm to assess efficacy as compared to percutaneous biopsy. A multicentric randomized study is ongoing to compare EUS-LB with percutaneous radiology-guided biopsy, with preliminary results indicating higher sample adequacy with EUS-LB than with percutaneous liver biopsy [19]. Also, in the present study, there was heterogeneity regarding needle type, needle size, and suction technique, making generalizability difficult. Lastly, we could not provide a cost-efficacy analysis.
To conclude, the present preliminary study showed a high diagnostic outcome and safety profile with the EUS-LB technique. EUS-LB can achieve excellent histological yield when performed with optimal technique. The presence of ascites may lead to suboptimal sampling. Patients undergoing endoscopic evaluation for varices under sedation will benefit from EUS-LB in the same setting. Moreover, patients with regional or patchy disease involvement requiring bilobar sampling can be considered for EUS-guided sampling. Ultimately, standardizing the EUS-LB technique and patient selection and ongoing multidisciplinary collaboration will be critical to widespread adoption.






