Introduction
Chronic urticaria (CU) is a disease that lasts more than 6 weeks, characterized by recurrent itchy wheals and/or angioedema [1]. CU shows both autoimmune and allergic disease characteristics, which is associated with an imbalance between cytokines and T lymphocyte subgroups [2]. Various data support the involvement of interleukins in the pathophysiology of CU [3, 4]. Interleukin (IL)-31 is mainly produced by T helper (Th) 2 cells, while it may also be produced by mast cells and dendritic cells, albeit less frequently [5, 6]. The IL-31 receptor is mainly expressed in skin and endothelium for regulating tissue responses and also tissue remodelling [7]. Moreover, IL-31 has also been described to contribute to itching via activation of the IL-31 receptor on sensory nerve cells [8]. IL-33 is a kind of alarmins that are released in response to cellular damage, apoptosis or immune activation. Its receptor ST2 is an IL-1R-related protein expressed on Th2 cells, mast cells, basophils and eosinophils [9, 10]. Consequently, IL-33 plays a crucial role in Th2-mediated immune responses, such as asthma, parasitic infections, and atopic dermatitis [11, 12].
In this regard, IL-31, and IL-33 might be involved in the pathogenesis of CU, and their levels could be a biomarker of disease severity or treatment response in CU. So far, there have been little available data regarding behaviour of IL-31, and IL-33 in patients with CU.
Aim
The aim of this study was to measure the values of plasma IL-31 and IL-33 levels in patients with CU and analyse their relations to disease severity, variables and treatment with omalizumab.
Material and methods
Inclusion and exclusion criteria of patients
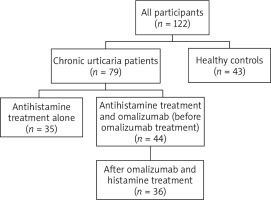
Seventy-nine CU patients admitted to the allergy outpatient clinic of Adana city hospital between October and December 2020 were enrolled. Patients aged between 18 and 65 years old were included. Pregnant, cancer those using immunosuppressive drugs, liver and kidney failure were not included in the study. Age- and sex-matched forty-three healthy volunteers were included in the study as a control group.
Urticaria activity scores (UAS) of 79 CU patients were recorded at the time of admission. Complete blood counts were done. Extra blood was taken from patients and the control group for cytokine IL-31 and IL-33 evaluation. Patients were followed up. Omalizumab was started in patients with high UAS7 despite taking 4-fold antihistamine drug therapy. According to guidelines, omalizumab was administered subcutaneously at 300 mg once a month for the treatment of antihistamine drugs resistant to CU [1].
Thirty-five patients with low UAS7 who responded well to antihistamine medication continued with only antihistamine medication. Omalizumab was given to 44 patients in addition to antihistamine drugs to control the disease. As a result, while all patients were receiving antihistamine medication, omalizumab was added to the treatment of only those who were resistant to treatment. Eight patients of 44 omalizumab receiving CU patients were lost to follow-up; as a result, their cytokine levels could not be determined after omalizumab treatment. IL-31 and IL-33 results of 36 CU patients receiving omalizumab were recorded.
Acquisition of study data
Demographic characteristics, laboratory results, skin prick test results, comorbid diseases were obtained from the patients’ files. Laboratory results were complete blood count, thyroid peroxidase enzyme antibody (anti-TPO), total immunoglobulin (Ig) E, Phadiatop (ImmunoCAP 100), vitamin D, vitamin B12 and D-dimer. We measured all of the laboratory parameters except D-dimer by using Unicel DxH 800 Cellular Analysis system (Beckman Coulter, Brea, CA, USA). D-dimer was measured by CS-2500 Sysmex.
A tube of blood was collected from patients and volunteers for studying the IL-31 and IL-33 cytokines, centrifuged and stored at –80°. ELISA method was used after the blood collection was fully completed. Biotek brand Elx50 model washer, and next level brand Alisai model reader was used. Test unit was reported in terms of pg/ml.
All of participants and patients had normal erythrocyte sedimentation rate and C-reactive protein levels. All patients were on antihistamine drugs immediately before the blood collection. Omalizumab was given to patients who are resistant to antihistamine drugs, and their blood was collected within 4–6 months of the initiation of omalizumab treatment; their post-treatment cytokine levels were recorded. The flowchart of the study is given in Figure 1.
Skin prick test
In the last 10 days, a skin prick test was performed for those who did not take antihistamine or steroid drugs and those who did not have an acute infection. Pregnant women with CU were excluded. Mixtures of mites and moulds, cat, dog, cockroaches, grass, cereals, tree pollen, weed mixture antigens were used in skin prick tests (ALK, Allergo). It was evaluated 15–20 min after the skin prick test was performed. The two largest diameters that cut each other perpendicularly were measured. If the result was more than 3 mm compared to the negative control, the test was accepted as positive [13].
Autologous serum skin test (ASST)
Five ml venous blood taken from patients was allowed to coagulate at the temperature room for 30 min. Serum was separated by centrifugation at 500 g in 15 min by creating a 45° angle with insulin or tuberculin injector; 0.05 ml serum was administered intradermally. As a negative control (same dose), sterile saline was applied in the same way. Thirty minutes after the injection, the diameter of the erythematous papule was measured. The diameter 1.5 mm larger than negative control was accepted as positive. An autologous serum test is often used to show circulating antibodies that cause urticaria [14].
Urticaria activity score (UAS7)
It is used to evaluate the severity of urticaria. It includes the number of swelling and itching severity daily for 7 days. UAS7 score ≤ 6 means well-controlled, 7–15 mild, 16–27 medium, 28–42 severe disease [1].
Statistical analysis
NCSS (Number Cruncher Statistical System) program was used for statistical analyses. Complementary statistical methods (average, standard deviation, median, frequency, percentage, minimum, maximum) were used for evaluating the study data. Quantitative data’s conformity to normal distribution was tested through the Shapiro-Wilk test. Student-t test was used for comparisons between two normally distributed groups and Mann-Whitney U test was used for comparisons between two non-normally distributed groups. Kruskal-Wallis test was used for comparisons between three or more groups that did not show normal distribution. Wilcoxon signed-rank test was used for intragroup comparisons of quantitative variables. Pearson χ2 test and Fisher’s exact test were used for comparison of qualitative data. Spearman correlation analysis was used for evaluation of relationships between quantitative variables. Statistical significance was deemed as p < 0.05.
Results
A total of 122 people, 79 patients and 43 controls, were included in the study. While 72.2% (n = 57) of the patient group were female, this rate was determined as 74.4% (n = 32) in the control group. The mean age of the study participants was 39.3 ±11.4 years, while the mean age was 39.6 ±11.2 years in the patient group and 38.8 ±11.9 years in the control group (p = 0.707). The median duration of disease was determined to be 36 months (range: 3 to 132 months). While 44 (55.7%) patients of the patient group received omalizumab and antihistamine drugs, 35 (44.3%) patients received only antihistamine treatment.
Angioedema was detected in 57.0% of the patients (n = 45). The rate of patients showing dermographism findings was 39.3% (n = 48), while the rate of patients with a positive ASST was 24.6% (n = 30). The patients’ urticaria activity score UAS7 ranged from 7 to 42, with a median of 36.0. When the patients were grouped according to their UAS7, 4 (5.1%) patients were found to have mild, 3 (3.8%) patients were found to have moderate, and 72 (91.1%) patients were found to have severe disease.
No statistically significant difference was detected between the groups in terms of ferritin, vitamin B12, antiTPO and vitamin D values of the patients (p > 0.05). Total IgE and D-dimer values of the patient group were detected as significantly higher, when compared with the control group (p < 0.001, p < 0.001, respectively). IL-31 values were found to be higher in patient group than in the control group and the difference was statistically significant (p < 0.001). There was no statistically significant difference in IL-33 values between CU patients and controls (p > 0.05) (Table 1).
Table 1
Biochemistry and interleukin values of patient and control groups
According to dual comparisons made to determine the difference, the IL-31 values of the control group were detected to be statistically significantly lower when compared with patients taking only antihistamine drugs, and patients taking both antihistamine drugs and omalizumab (p = 0.009; p = 0.002; p < 0.001). When the IL-31 values of those receiving omalizumab treatment were compared before and after the treatment, no statistically significant difference was found between the groups (p = 0.100) (Table 2).
Table 2
Interleukin levels according to treatment groups
Interleukin-33 values of CU patients receiving omalizumab treatment before and after the treatment do not show a statistically significant difference (p > 0.05).
The change of IL-31 values in CU patients receiving antihistamines and omalizumab, when compared with patients receiving only antihistamines does not display a statistically significant difference, regarding gender, age, illness duration of patients (p > 0.05). Before and after omalizumab treatment, IL-31 values do not display a statistically significant difference as to the presence or non-presence of angioedema and allergic diseases in patients (p > 0.05). No statistically significant difference between the IL-31 values before and after omalizumab treatment was detected regarding patients suffering from angioedema and patients not suffering from angioedema (p > 0.05). No statistically significant difference between the IL-31 values before and after omalizumab treatment was detected regarding patients suffering from allergic disease and patients not suffering from allergic disease (p > 0.05). No statistically significant difference between the IL-31 values before and after omalizumab treatment was detected according to the ASST, DPT, UAS7 values of patients (p > 0.05) (Table 3).
Table 3
Relationship between IL-31 level and study parameters
No statistically significant relationship was detected between the patients’ leucocyte, neutrophil, lymphocyte, NLR, eosinophil, ferritin, vitamin B12, D-dimer, anti-TPO, and vitamin D values, and IL-31 values (p > 0.05). IL-31 values of patients before and after omalizumab treatment do not display a statistically significant difference in terms of total Ig E levels (normal/high) (p > 0.05) (Table 4).
Table 4
Comparison of complete blood count parameters of the participants
Discussion
With this study, we share the result that IL-31 was independently elevated in omalizumab treatment in CU patients receiving antihistamines, while IL-33 did not show a difference. CU pathogenesis is multi-factorial, and there are studies indicating the involvement of IL-31 and IL-33 cytokines in the pathogenesis [15]. In the study we carried out with CU patients, while serum IL-31 level was detected to be higher in a statistically significant manner in these patients, when compared with healthy volunteers, no statistically significant difference was detected regarding the serum IL-33 level. When patients receiving only antihistamine drugs during treatment, and patients receiving both antihistamine drugs and omalizumab during treatment were compared with the control group, the serum IL-31 level was found to be higher in a statistically significant manner in the patients. Besides, the IL-31 levels of those receiving omalizumab, which are higher in a statistically significant manner when compared with the control group before the treatment, were detected to be still higher in a statistically significant manner when compared with the control group 4–6 months after the treatment.
The most well-known itch mediators were histamine and neuropeptides during the past years; however, new studies showed that IL-31 cytokine also has a role [7]. It had been detected that the inflammation in the skin is correlated with the increased number of basophils, in CU [16].
It had been shown that IL-31 was strongly expressed in the skin of patients suffering from CU, and was released from basophils following anti-IgE, IL-3 or fMLP stimulation [17]. In another study, Raap et al. have shown that the serum IL-31 level was also increased [18]. There are also other studies, which show that the serum IL-31 level is high in patients suffering from CU [2]. Our study, in line with the literature, also found that the IL-31 level was high, regardless of the UAS7 score and the treatment received by the patients. In studies carried out with 15 patients who went into full remission with 6-month omalizumab treatment, the patients’ treatment the median IL-31 level was significantly (p = 0.004) reduced by 48% [19]. In our 36 CU patients receiving omalizumab and antihistamine drug, no reduction was detected in the IL-31 level with omalizumab. We may have obtained a different result, since our number of patients was higher and/or we observed the serum/cytokine levels earlier when compared with patients receiving omalizumab, or we did not evaluate the treatment responses. Since CU is an undulating condition, it is possible for the measured plasma IL-31 not to reflect the truth. In another recent research omalizumab significantly reduced the IL-31 levels of the patients suffering from CU, when compared with placebo. Nevertheless, there was no correlation between the IL-31 level and urticaria activity score, wheals or itch scores [19]. In another study, conducted by Raap et al., in which the serum IL-31 level of CU patients was found to be higher in a statistically significant manner when compared with the control group members, no statistically significant difference was found between the serum IL-31 levels of patients with positive and negative ASST, a result in line with our study [18]. While we did not make a separate evaluation for patients regarding itch, the high average UAS may also mean that the itch level is high, and in consequence, a higher serum IL-31 level may have been detected in the patient group, when compared with the control group. According to the study published in 2013, no significant correlation was detected between UAS7 severity and any of the biomarkers evaluated in the comprehensive ELISA analyses, which also included IL-31 and IL-33 [20].
IL-33 is known as an inflammatory cytokine. It is presumed that it may play an important role in the development of inflammatory reactions in CU, for the release of Th2-mediated cytokines such as IL-33, IL-5, and IL-13 may eventually increase the number of eosinophil and total IgE level [21]. The reason why the total IgE and eosinophil levels in our CU patients were found to be higher in a statistically significant manner when compared with the control group, may be the effect of the IL-33 cytokine, regarding which no statistically significant difference was detected between the patients and the control group [22]. However, there are studies showing a high level of IL-33 in skin biopsy material [23], while no skin biopsy was performed within our study. In the study conducted with 51 CU patients, it was detected that the IL-33 level was higher in a statistically significant manner, when compared with the control group, and that the IL-33 level in severe patients was higher in a statistically significant manner, when compared with the mild patients [2]. In another study, an important reduction was shown in the IL-33 level of CU patients receiving 2-week desloratadine treatment, and it is presumed that drugs affect the plasma level [24]. Since all the CU patients included in our study were receiving antihistamine drug treatment (mostly desloratadine, rupatadine, cetirizine) immediately before the blood collection, their IL-33 levels may have decreased. It may be the reason of the lack of statistically significant difference between the patient and control group in terms of the IL-33 plasma level. The reason of the difference may be the high number of patients, and genetic differences.
The minimum requirement for a disease biomarker is a correlation with disease activity. The most promising candidates so far are D-dimer, CRP, IL-6 and MPV, whose levels or values were significantly higher in more severe CU in 10, 7, 5, 4 and 3 studies from different investigators, respectively. In studies showing the relationship between disease activity and D-dimer, a strong correlation was found. The more severe the disease activity, the higher the D-dimer level was found. D-dimer has even been shown to decrease with treatment [25].
Kessel et al. [26] showed that high levels of total IgE were significantly associated with more severe CU as measured by ELISA with a cut-off of 175 U/ml. In contrast, Baek et al. [27] used immunoradiometric assay with a cut-off of 91 IU/ml and found no association between total IgE and CU severity. In our study, when we compared the levels before and after omalizumab treatment, IL-31 measurements of patients according to total IgE levels (normal/high), we did not detect a statistically significant difference. We may have obtained different results, since we defined the cut–off values differently according to our own laboratory.
Asero et al. have demonstrated that D-dimer plasma levels parallel the clinical response to omalizumab [28]. D-dimer is a good marker of disease activity in most patients with CU, it may be used in the follow-up of treatment response [26]. In our study, we detected a high D-dimer level, as it is known in CU levels; however, no evaluation was made since its level with omalizumab treatment was not observed.
In our study no statistically significant difference was detected between the patient and control groups in terms of vitamin D levels. Because we did not analyse factors that affect the levels of vitamin D (e.g. smoking, daily sunlight exposure per subject, baseline nutritional status, time of blood sampling) and taking vitamin D replacement treatment due to the design of the study.
Significant association was shown between levels of D-dimer and IL-31 and response to treatment with cyclosporine and omalizumab, respectively. Up to 40–50% of patients with CU have autoreactive urticaria as defined by positive ASST.
Conclusions
While we detected a high level of IL-31 in CU patients, when compared with the control group, we detected a similar level of IL-33 between the two groups. However, when we observed the relationship of the highest level of IL-31 we detected with patients’ clinical and laboratory variables, we did not detect a significant relationship. We were unable to re-observe these variables after the treatment due to the design of our study, therefore we were unable to make an evaluation of the same. Since new therapies are still needed in the treatment of CU, more cytokine-related studies are needed.