Introduction
Omalizumab is a monoclonal IgE antibody that is used in patients with chronic spontaneous urticaria (CSU) resistant to conventional drugs. Patients with chronic idiopathic urticaria typically have low serum dehydroepiandrosterone sulphate (DHEA-S) levels. This study investigated the effect of the drug on serum DHEA-S levels and its correlation with the remission and relapse times of CSU.
In chronic urticaria, the mechanism of action of this biological agent has not been fully explained [1–3]. In clinical observations and series, the efficacy and remission times in patients were found to be variable. In the literature, studies investigating drug activity discrimination and remission variability are scarce. In addition, there is no single laboratory parameter that is predictive for drug efficacy and could be used for follow-up.
It is known that serum DHEA-S levels are low in patients with CSU, which may be due to stress or other factors in the neuroendocrine system. Because sex hormones alter during the menstrual cycle, female patients may develop hives in response to pregnancy, menopause, oral contraceptive use, and hormone replacement treatments [4, 5]. DHEA and DHEA-S are precursors for sex hormones. Circulatory DHEA and DHEA-S levels are associated with anti-inflammatory, anti-proliferative and immunomodulatory events [6–8]. Thus, monitoring changes in the serum level can aid in the diagnosis and treatment of CSU.
To the best of our knowledge, this is the first study to investigate the effect of omalizumab treatment on DHEA-S levels in CSU patients. Therefore, we think that it is important to investigate the effect of omalizumab treatment on serum DHEA-S levels in patients with CSU.
Aim
This study was aimed to investigate the effect of the drug on DHEA-S serum levels and its correlation with remission and relapsing times in patients with CSU.
Material and methods
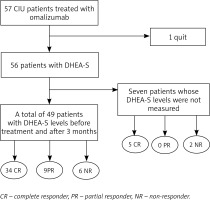
This study included 57 patients with CSU who were referred to the Dermatology Department of Sağlık Bilimleri University, Kayseri Training and Research Hospital, and 28 healthy volunteers. The inclusion criteria in the patient group were as follows: patients currently prescribed anti-histaminic, leukotriene antagonist and cyclosporine treatments but did not receive systemic steroid therapy within prior 6 months. The exclusion criteria included endocrine and metabolic diseases, hormone replacement therapy and the use of any drug likely to affect serum hormone levels (Figure 1). Demographic data including age and gender as well as complaints related to any chronic illness of the patients were recorded in both patient and control groups. Blood samples for baseline DHEA-S measurements were obtained between 8 and 9 a.m. after overnight fasting.
Following blood sampling, 300 mg omalizumab was administered to the patient group via subcutaneous injection. Omalizumab injections were repeated – one injection per week for 24 weeks. One patient in the study group left the study after the third dose; thus, the patient was excluded from analysis, and data from 56 patients were used in final analyses.
Patients who achieved remission following all six injections were referred as the complete remission (CR) group; those who did not respond were referred as non-responders (NR) group, while those with partial improvement were classified into the partial response (PR) group. The time between the six injections and the next urticaria episode was defined as duration of remission in the CR group. Patients who reported urticaria at the end of this period were classified as relapses. The relationships between the DHEA-S levels of patients and the relapse rates, treatment response and remission times were also investigated.
The DHEA-S levels of healthy participants in the control group were compared with the pre-omalizumab DHEA-S measurements of patients with CSU. The DHEA-S values of the patient group were also examined at baseline and 12 weeks after onset of omalizumab treatment. However, seven patients were lost to follow-up after 3 months. This analysis was conducted on 49 patients including 34 patients with CR, 9 patients with PR and 6 non-responders.
Statistical analysis
The Shapiro-Wilk test and histograms were used to assess normal distribution. In the intergroup comparisons, the variables with normal distribution were assessed by using Student’s t test while those with skewed distribution were assessed by the Mann-Whitney U test. The Wilcoxon test was used to compare the data from different time points within the group. The χ2 test was used to compare categorical variables. Pearson’s correlation test was used to assess the correlation among continuous variables. Continuous variables were expressed as the mean ± standard deviation or median (min-max) where appropriate. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 21.0 (SPSS Inc., Chicago, IL, US).
Results
Comparison of the age, gender and serum DHEA-S values in patient and control groups
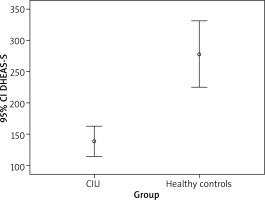
The mean age was 39.89 ±10.79 years in 56 patients with CSU. There were 40 female and 16 male patients in the study group. The mean age was 35.9 ±7.75 years in the control group including 8 male and 20 female volunteers. There was no significant difference between the control and patient groups regarding age (p = 0.088) or gender (p = 0.345). The median value for the complaints was 48.0 (range: 3–300) weeks. The median baseline serum DHEA-S value was 278.1 (range: 129.7–612.1) µg/dl in the control group whereas it was 116.7 (range: 21.5–448.7) in the patient group, indicating a significant difference between groups (p < 0.001) (Figure 2).
Comparison of the serum DHEA-S values of the CR and PR groups
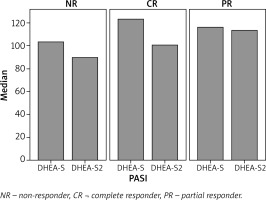
The serum DHEA-S values of 39 patients in the CR group and 8 patients in the NR group were 125.7 (range: 21.5–310.3) µg/dl and 103.4 (range: 34.4–321.1) µg/dl, respectively. The serum DHEA-S values were higher in the CR group than those in the NR group, but the difference did not reach statistical significance (p = 0.977).
Comparison of the serum DHEA-S values in the relapse and remission patients in the CR group
During follow-up, relapses were noted in 74.4% (n = 29) of the 39 patients in the CR group. The median time to relapse was 12.0 weeks (range: 4.0–36.0). No relapse was observed in 10 (25.6%) patients. The median remission time was 18.0 (range: 8.0–28.0) weeks in these patients.
The median serum DHEA-S values were 113.2 (range: 32.0–310.3) µg/dl in patients without relapse whereas they were 125.7 (range: 21.5–299.6) µg/dl in those who experienced relapse but the difference did not reach statistical significance (p = 0.723).
NR group serum DHEA-S values
There were 8 patients in the NR group. The median DHEA-S value before treatment in these patients was 103 (range: 4.4–321.1) µg/dl.
Comparison of the age, sex, duration of disease and baseline serum DHEA-S values between CR group and the PR plus NR groups
The mean age was 39.2 ±11.4 years in the CR group while it was 41.4 ±9.3 years in remaining patients (NR plus PR) (p = 0.568). The disease duration was 48.0 (range: 3–240) weeks in the CR and 72 (range: 6–300) weeks in the NR + PR group. There was no statistically significant difference in terms of the disease duration between the two groups. In the PR + NR group, there was 1 (5.9%) male and 16 (94%) female patients. In the CR group, male participants made up 38.5% (n = 15), while females represented 61.5% (n = 24). There was a statistically significant difference between the groups (p = 0.013). The serum DHEA-S values were 125.7 (range: 21.5–310.3) µg/dl in the CR group and 112.0 (range: 34.4–448.7) µg/dl in the NR + PR group (p > 0.05).
Comparison of the DHEA-S values obtained at baseline and 12 weeks after omalizumab treatment between the patient and control groups
The serum DHEA-S values 12 weeks after the start of treatment could be determined in only 49 patients. Of 49, 34 patients were in the CR, 9 were in the PR and 6 were in the NR group. The median DHEA-S value at baseline was 116.3 (range: 21.5–448.7) µg/dl. The median DHEA-S value measured after 3 months was 98.4 (range: 10.0–410.0) µg/dl. The decrease was statistically significant (p = 0.003) (Figure 3).
There was also a decrease in the DHEA-S values in 6 patients in the NR group and in 9 patients in the PR group for whom measurements were available 12 weeks after the start of treatment, but these values were not statistically significant (112.0 (range: 34.4–448.7) µg/dl and 97.2 (range: 10.0–410.0) µg/dl, p = 0.057).
There was a significant difference between DHEA-S levels obtained at baseline and 12 weeks after treatment in 34 patients in the CR group The median DHEA-S value was 123.1 (range: 21.5–299.6) µg/dl at baseline. After 3 months, it had decreased to 100.4 (range: 23.1–301.9) µg/dl (p = 0.021).
The serum DHEA-S values of 9 patients with remission in the CR group, in which the last DHEA-S measurements could be performed, were found to be significantly decreased following treatment (96.6 (range: 32.0–273.3) µg/dl and 74.7 (range: 26.7–203.4) µg/dl, p = 0.028, respectively). There were no significant differences between the serum values of the 25 patients with relapse in the CR group either before or after the treatment 125.7 (range: 21.5–299.6) µg/dl and 113.3 (range: 23.1–301.9) µg/dl, respectively, p = 0.183) (Table 1).
Table 1
The circulating DHEA-S levels of patients with chronic idiopathic urticaria treated with omalizumab
Correlations
No statistically significant correlation was found between the serum DHEA-S values and disease duration, age, remission time, duration of the onset of remission or recurrence times. There was also no statistically significant correlation in the CR group, the PR group or the NR group. When the CR group was considered, there was a statistically significant negative correlation between disease duration and the serum DHEA-S levels (r = –0.377, p = 0.018).
Discussion
It is known that hormonal changes play a role in the aetiology of CSU. In particular, an increase in oestrogen and progesterone levels may be closely related to the appearance of hives [9]. DHEA and DHEA-S play an important role in both humoral [10] and cellular [8, 11] immunoreactivity. DHEA-S also factors into important immunological and inflammatory reactions, and variations in serum DHEA-S concentrations have been described in several chronic inflammatory diseases [12–14]. In particular, a DHEA-S deficiency is a persistent feature in some autoimmune diseases and is a significant contributor to the aetiology and/or pathophysiology of these conditions [15, 16]. However, it is difficult to find clear evidence that low concentrations of hormones directly contribute to the development of some systemic diseases [17].
Low serum DHEA-S levels are more common in patients with CSU. One explanation for this correlation is physiological distress [18]. During a chronic inflammatory response, hormonal shifts may be impaired and the release of DHEA and DHEA-S may be reduced, as opposed to the increased cortisol levels that might be expected [16, 17]. DHEA, the precursor of DHEA-S, has been used successfully in the treatment of hereditary angioedema due to its inhibition of complex activation [19].
In this study, the relationship between CSU and DHEA-S serum levels was assessed following treatment with omalizumab, a monoclonal IgE antibody. DHEA-S levels in the group of CSU patients were lower than those of healthy volunteers, which was consistent in the literature.
When 39 patients with complete remission were evaluated, the baseline DHEA-S values were higher than the values for the whole group (125.7 (range: 21.5–310.3) µg/dl vs. 116.7 (range: 21.5–448.7) µg/dl). The median DHEA-S value of the 8 patients with no response to treatment was 103 (range: 34.4–321.1) µg/dl. Based upon these results, having a higher DHEA-S level at baseline may suggest that there is an advantage in entering remission, but there was still no statistical significance. Larger studies will be required to determine if an expansion of the sample size can make this relationship statistically significant.
When all 56 patients in the study were assessed, the omalizumab treatment significantly lowered their DHEA-S levels, which were already low compared to the population’s baseline values. The median DHEA-S value before treatment was 116.3 (range: 21.5–448.7) µg/dl, while the median DHEA-S value measured after 12 weeks was 98.4 (range: 10.0–410.0) µg/dl. Before treatment, the median DHEA-S value was 123.1 (range: 21.5–299.6) µg/dl; after 3 months, it was decreased to 100.4 (range: 23.1–301.9) µg/dl.
The DHEA-S levels of 9 patients still in remission were statistically significantly lower than their baseline values after 3 months. In the 25 patients who experienced a relapse, the decrease in DHEA-S values after 3 months was not statistically significant. Their value at baseline was 125.7 (range: 21.5–299.6) µg/dl, and this value was decreased to 113.3 (range: 23.1–301.9) µg/dl after 3 months.
The median DHEA-S value at baseline for the 9 CR patients who reported no relapse and had post-treatment DHEA-S measurements was 96.6 (range: 32.0–273.3) µg/dl at baseline; these participants had low baseline values compared to those who entered remission and also those who did not respond. After treatment, the level was significantly decreased to 74.7 (range: 26.7–203.4) µg/dl.
When we evaluated the 6 patients who did not respond to omalizumab at all and the 9 patients who responded but did not achieve complete remission as a single group at baseline, the median DHEA-S value prior to omalizumab was 112.0 (range: 34.4–448.7) µg/dl. After 3 months, the median DHEA-S value was 97.2 (range: 10.0–410.0) µg/dl. Examination of all these data revealed that the patients who entered remission started out with higher DHEA-S values. In addition, serum DHEA-S levels were significantly reduced by treatment with omalizumab. Interestingly, the decrease in the DHEA-S values of patients who reported relapses was not significant.
It is not easy to interpret these results using our current knowledge. While some prior studies have concluded that a DHEA-S deficiency sets the groundwork for inflammatory processes [6–8, 10], others have reported that an increase in DHEA-S is considered protective for chronic urticaria. This information is contradictory and may be confusing for patients.
Although it is impossible to explain these declines, some theories can be proposed. Inflammation is suppressed by treatment with omalizumab, and steroidogenesis may also be slowed. In relapsed patients, the failure to suppress inflammation may be responsible for the absence of a decrease in steroidogenesis. This theory should be tested in future studies and proven using data.
Omalizumab has been successfully used in the treatment of chronic idiopathic urticaria. It is also effective for both autoimmune and non-autoimmune chronic urticaria as well as physical and cholinergic urticaria [20]. Omalizumab can be used in some diseases, such as atopic dermatitis, food allergies and asthma, where serum IgE levels are elevated, when patients do not respond to other treatments. Omalizumab, a monoclonal IgE antibody, demonstrates its effect by reducing free IgE levels in the plasma. The recommended omalizumab dose in chronic urticaria is 75–375 mg every 2 or 4 weeks based upon the weight of the patient and also the serum IgE levels. Ferrer et al. reported that 300 mg/month would be the most appropriate dose [21], while Ivyanskiy et al. stated that the minimum dose should be 150 mg [22]. After reviewing 49 double-blind, placebo-controlled studies of disease, Maurer et al. recently suggested that omalizumab treatment should be continued over 6 months [23]. According to many reports, omalizumab is a well-tolerated drug. The most serious side effect is the risk of anaphylaxis, while the biggest disadvantage is its cost.
The reasons for low serum DHEA-S levels in urticaria remain unknown. According to some hypotheses, adrenal gland DHEA-S production may be affected by a chronic inflammatory response and the effects of severe stress [16, 17]. DHEA-S is the precursor to sex hormones and is the most common steroid hormone in serum. DHEA-S reaches its highest level in the third decade of life, and then serum DHEA-S levels begin to fall in reverse proportions as age increases [12, 24, 25].
Many skin diseases can occur as a result of negative stress, including both the first onset and any exacerbation of chronic idiopathic urticaria [26]. Itching from urticaria and a feeling of discomfort may also arise in many stressful situations [27]. Baiardini et al. reported that chronic urticaria can impair one’s quality of life at very severe levels [28].
Increasing the quality of life of urticaria patients with omalizumab and similar treatments may alter their levels of sex hormones, such as DHEA-S, by acting on the hormonal balance. The decrease in the serum DHEA-S levels from a mean of 125.7 (range: 21.5–299.6) µg/dl at baseline to an average of 113.3 (range: 23.1–301.9) µg/dl after the third month of omalizumab treatment was also statistically significant. However, there was no statistically significant difference between the DHEA-S level reduction and the duration of the chronic idiopathic urticaria remission or relapse. This statistically insignificant outcome was not different between the participants in the complete remission (123.1 (range: 21.5–299.6) µg/dl, n = 34) group or the partial remission (116.3 (range: 57.9–448.7) µg/dl, n = 9) group.
Conclusions
This study examined the effects of omalizumab therapy on the serum DHEA-S levels in 56 patients with chronic idiopathic urticaria using their remission and relapse times. We measured their serum levels before the initial treatment and again 3 months after the treatment began. The DHEA-S levels were significantly lower following omalizumab therapy but were not related to the remission and relapse times. Omalizumab treatment may also affect the levels of sex hormones.
To the best of our knowledge, this was the first study to examine the effect of omalizumab treatment on DHEA-S levels in chronic idiopathic urticaria patients. Since DHEA-S is known to be associated with anti-inflammatory and immunomodulatory effects, we were expecting an elevation in serum DHEA-S levels after the treatment, but the results obtained were just the opposite. The DHEA-S levels were found to be significantly lower after omalizumab therapy but were not related to patients’ remission and relapse times. This subject will be better elucidated by further studies.