Dupilumab, a biologic agent approved for chronic moderate-to-severe atopic dermatitis (AD), effectively inhibits interleukin (IL) 4 (IL-4) and IL-13 signalling [1]. However, its potential adverse effects warrant attention. Herein, we report the case of generalized pustular psoriasis (GPP) following a 2-month course of dupilumab therapy.
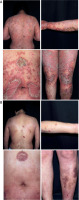
A 50-year-old Chinese woman presented with a widespread pruritic rash and pustules persisting for 40 days. She had a prior diagnosis of AD, confirmed by recurrent eczema-like eruptions, elevated IgE levels and eosinophils according to the guidelines [2]. Treatment with dupilumab (600 mg initial dose followed by 300 mg every 2 weeks) was initiated. After the first injection, her AD symptoms improved significantly. However, following the second dose, scattered patches of redness and pustules developed on her trunk and limbs. These lesions worsened after the third dose, progressing to full-body involvement (Figure 1 A). Dupilumab was discontinued.
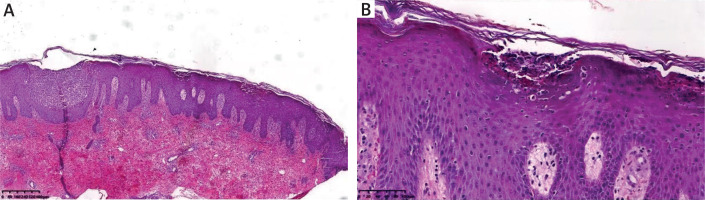
Pustule cultures were negative for bacterial growth. Pathological examination revealed epidermal hyperkeratosis with hypokeratosis, Kogoj microabscesses, thickening of the acanthotic layer, large pustules below the stratum corneum, and significant amounts of central granulocyte infiltration in vesicular cavities (Figure 2). Based on clinical and pathological findings, GPP was diagnosed. The patient was started on oral acitretin (40 mg/day), leading to lesion resolution and pruritus improvement within 2 weeks (Figure 1 B). The dose was tapered to 30 mg/day after 2 weeks, then 20 mg/day after 4 weeks. Acitretin was discontinued after 2 months with no recurrence of AD or GPP during a 1-year follow-up.
Several potential mechanisms may explain this paradoxical reaction: dupilumab blocks the IL-4/IL-13 signalling pathway, potentially leading to excessive activation of the IL-23-Th17-IL-17 pathway [3]. Dupilumab may inhibit the activation and expression of IL-36 and subsequently inducing pustular psoriasis [4].
There are several cases reported GPP after dupilumab treatment in 5 years [5–13] (Supplementary Table S1). This case underscores the complexity of managing dermatological conditions. While dupilumab is effective for AD, it may inadvertently trigger GPP in rare instances. Acitretin proved successful here, emphasizing the need for individualized therapy. Further research is needed to elucidate risk factors and optimize treatment strategies for such paradoxical reactions.