Introduction
Prurigo nodularis (PN) is a chronic pruritic and inflammatory skin disorder characterized by symmetrically distributed, often on the extremities and occasionally on the trunk, keratotic papules and nodules accompanied by intense and unbearable itching [1]. The aetiology of PN remains unclear; however, it can be triggered by various factors including skin diseases, systemic conditions, and neuropsychological factors, with their interactions exacerbating the skin lesions [2]. Type 2 inflammatory responses are implicated in the pathogenesis and progression of the disease [3].
The treatment options for PN are diverse, and there is no consensus on the standard treatment regimen. Therapeutic approaches include both systemic and non-systemic treatments [4]. Non-systemic therapies, considered first-line, encompass topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), and intralesional corticosteroid injections. However, the prolonged use of TCS may lead to adverse effects such as skin atrophy, telangiectasia, acne, and folliculitis [5]. Systemic treatments include gabapentinoids, antidepressants, immunosuppressants, and biological agents such as dupilumab and nemolizumab [6]. Methotrexate [7] and thalidomide [8] have also been reported to effectively alleviate inflammation and pruritus, but their long-term use is limited by adverse effects. Dupilumab received FDA approval for PN in September 2022 and has demonstrated significant efficacy in reducing pruritus and skin lesions with rare associated side effects [9]. A phase 3 clinical trial suggested that nemolizumab had good efficacy in the treatment of PN [10], and it was approved by the FDA for the treatment of PN in August 2024. However, nemolizumab has yet to obtain the approval for marketing in China.
Dupilumab is a fully human monoclonal antibody that targets the alpha subunit of the IL-4/13 receptor. By blocking the biological activities of IL-4 and IL-13, dupilumab inhibits type 2 inflammatory responses [11]. Since IL-4 and IL-13 are implicated in the pruritic mechanisms associated with PN, dupilumab may offer therapeutic benefits for this condition. Dupilumab has been approved in China for the treatment of moderate to severe atopic dermatitis in patients aged 6 years and older.
Aim
This study retrospectively evaluates the efficacy and safety of dupilumab in managing moderate to severe PN, and the results are summarized as follows.
Material and methods
Study population
A total of 76 patients diagnosed with moderate to severe PN and treated with dupilumab at the Dermatology Outpatient Clinic of the Second Affiliated Hospital of Soochow University from March 2021 to June 2023 were included in the study. Diagnostic criteria: (1) presence of refractory nodular skin lesions; (2) pruritus duration of ≥ 6 weeks; and (3) history and/or signs of repeated scratching, picking, or rubbing. Inclusion criteria: (1) meeting the above diagnostic criteria and confirmed PN diagnosis; (2) disease severity assessed as moderate to severe by the same primary dermatologist, with Investigator’s Global Assessment (IGA) ≥ 3 and Numerical Rating Scale (NRS) ≥ 4; (3) age ≥ 18 years at the time of initial treatment; (4) history of inadequate response to traditional PN treatments; and (5) signed informed consent for dupilumab treatment and good compliance, with all patients completing the 52-week treatment. Exclusion criteria: (1) pregnant and lactating patients; (2) patients with active systemic infections such as parasitic infections, hepatitis, tuberculosis, syphilis, or HIV; (3) patients with progressive malignant tumours; (4) patients with other severe underlying diseases that could interfere with efficacy evaluation; (5) differential diagnoses such as nodular pemphigoid, skin tumours, or scabies; and (6) patients who had received systemic phototherapy, other biological agents, immunosuppressants, or small molecule drugs within 6 months before or during the treatment period.
Treatment protocol
Patients enrolled in the study received subcutaneous injections of dupilumab administered by trained professionals. The initial dose was 600 mg, given as two subcutaneous injections. Thereafter, 300 mg was injected every 2 weeks. After 16 weeks of treatment, the maintenance dose was adjusted based on the patient’s condition by the clinician, either continuing with 300 mg every 2 weeks or extending to 300 mg every 3–4 weeks, administered regularly. Dupilumab injection solution (Dupilumab), brand name: Dupixent®, manufactured by Sanofi, was provided in pre-filled syringes at a dosage of 300 mg (2.0 ml) per syringe, with two syringes per box.
During the course of dupilumab treatment, patients were allowed to concurrently use oral antihistamines, topical moisturizers, TCS, and TCI.
Study methods
The retrospective study collected general patient information from outpatient medical records. During and after the dupilumab treatment period, follow-up phone interviews were conducted by dedicated personnel. Data on the number of nodules, IGA, NRS, and Dermatology Life Quality Index (DLQI) scores were recorded at baseline and at weeks 4, 8, 16, 26, and 52 of treatment [12]. Adverse events post-treatment were also documented.
General information
Basic information included patient name, age, gender, and visit date. Medical history data encompassed age at initial onset, duration of the disease, diagnosis and treatment history, atopic history, past medical history, and drug allergy history.
Efficacy evaluation criteria
IGA Score: Severity was graded based on the number of prurigo nodules: Grade 0 (no lesions, 0 nodules), Grade 1 (almost clear, 1–5 nodules), Grade 2 (mild, 6–19 nodules), Grade 3 (moderate, 20–100 nodules), and Grade 4 (severe, more than 100 nodules).
NRS Score: Patients self-rated the intensity of the most severe itch experienced in the past 24 h on a scale of 0 to 10. A score of 0 indicated no itching, 1–3 indicated mild itching (not affecting sleep), 4–6 indicated moderate itching, 7–9 indicated severe itching (disturbing sleep), and 10 represented the worst imaginable itching.
DLQI Score: The DLQI was used to assess the impact of the skin disease on the patient’s quality of life over the past week. It included 10 items covering symptoms such as itching, soreness, pain, stinging, embarrassment, impact on shopping and housework, choice of clothing, social and leisure activities, sports, work or study, relationships, sexual activity, and the burden of treatment. Each item was scored from 0 to 3, with a total score range of 0–30. Higher scores indicated a greater impact on quality of life: 0–1 (no effect), 2–5 (mild effect), 6–10 (moderate effect), 11–20 (large effect), and 21–30 (extremely large effect).
Statistical analysis
Statistical analyses were performed using SPSS 29.0 software. Categorical data were expressed as percentages (%). The Shapiro-Wilk test was used to test the normality of continuous data. Normally distributed data were analysed using paired t-tests, while non-normally distributed data were analysed using the Wilcoxon signed-rank test. Data were described as mean ± standard deviation (SD), with a significance level set at p < 0.05.
Results
Baseline characteristics
This study successfully included 76 patients with moderate to severe PN. In 23 patients the diagnosis was confirmed by skin biopsy before treatment, and in all patients the diseases such as mycosis fungoides and bullous pemphigoid were excluded. Basic demographic information is provided in Table 1.
Table 1
Basic demographic information for patients with PN
Efficacy analysis
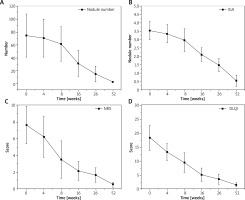
All patients completed the 52-week treatment. Dupilumab has shown significant clinical efficacy in the treatment of moderate to severe PN. During the initial 16 weeks, patients received a regular dosage of 300 mg every 2 weeks. After completing the 16-week treatment, patients with severe conditions continued with bi-weekly injections, while those with marked improvement extended the injection interval to every 3–4 weeks. There were significant reductions in the number of nodules, IGA scores, NRS scores, and DLQI scores at weeks 4, 8, 16, 26, and 52 of treatment (Table 2). These differences were statistically significant (Figure 1).
Table 2
Changes in various scores in PN patients after dupilumab treatment
This study meticulously documented the safety of dupilumab in treating moderate to severe PN. The primary adverse reactions observed during treatment included injection site reactions and facial erythema (Table 3). These reactions were mild in severity; injection site reactions resolved spontaneously and did not impact subsequent treatments after changing the injection site. Facial erythema was transient and improved with symptomatic treatment. Throughout the 52-week treatment period, no patients experienced serious adverse events requiring discontinuation of therapy.
Discussion
PN is often associated with severe pruritus and is resistant to conventional treatments. The intense itching compels patients to self-mutilate through scratching, resulting in disfigurement, depression, and significant impairment of quality of life. Patients with PN experience considerable deterioration in quality of life, with the vast majority (97.2%) trapped in an itch-scratch cycle, leading to sleep disturbances, anxiety, and depressive states due to the severe and unrelenting pruritus. Multiple lines of evidence have demonstrated that PN is associated with Th2 inflammatory immune responses [13, 14]. Various inflammatory cytokines are involved in the pathogenesis of PN, with increased expression of IL-4, IL-5, IL-10, IL-13, IL-17, IL-22, and IL-31 detectable in prurigo lesions [3, 15]. It is also related to neurogenic inflammation triggered by repeated scratching of the lesions. Studies have shown strong expression of p75NTR in the lesional skin of PN, indicating specific neuropathic involvement in PN. Additionally, cortistatin (CST) and substance P (SP) have been detected in the epidermis. The SP/MRGPRs pathway plays a significant role in the immune response and pruritus associated with PN [16]. There is also evidence suggesting that immune processes involving Th1, Th17, and Th22 pathways play a role in its development [2, 17]. Current evidence indicates that the JAK-STAT pathway is also involved in the pathogenesis of PN [18].
Traditional systemic therapies for PN such as thalidomide, glucocorticoids, and immunosuppressants like cyclosporine have shown limited clinical efficacy and significant potential toxic effects. These treatments may also impose a greater health burden on elderly patients with comorbid conditions [19–22]. With the emergence of new increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo therapeutic targets in recent years, novel therapies have gradually been developed. These include biologics such as dupilumab, nemolizumab, tralokinumab, and lebrikizumab, as well as various small molecule agents like neurokinin-1 receptor antagonists, Janus kinase inhibitors, and µ-opioid receptor antagonists/m-antagonists/k-agonists. These therapies have shown promising efficacy [23, 24]. However, except dupilumab and nemolizumab, the sample sizes for these treatments remain relatively small, and they have not yet been approved for the treatment of PN.
Since its approval for atopic dermatitis (AD), dupilumab has been widely utilized, demonstrating clear efficacy and safety [14, 25]. PN shares certain clinical and pathophysiological features with AD, which is considered one of the most relevant disease factors for PN. This shared pathophysiology likely underlies the effectiveness of dupilumab in treating PN [3, 26, 27]. Dupilumab functions by inhibiting IL-4 signalling through binding to type I and type II receptors and IL-13 signalling through binding to type II receptors, thus blocking Th2 cell-mediated inflammatory responses. This mechanism disrupts the distinctive itch-scratch cycle in PN, leading to clinical remission [28]. Therefore, we selected dupilumab for the treatment of moderate to severe PN and aimed to evaluate its efficacy and safety in this patient population.
We present real-world data from a 52-week follow-up study of 76 patients with PN who were treated with dupilumab at the Suzhou Hospital, China. The data indicate that dupilumab significantly improved both the clinical signs and manifestations of PN. The average age of onset for PN in our cohort was 50 years, with no significant gender differences noted, and the majority of patients had comorbid conditions.
Our findings demonstrate a reduction in Visual Analog Scale (VAS) scores from a baseline of 7.65 to 6.24 at the 4-week follow-up and further to 0.54 at the 52-week follow-up. DLQI scores decreased from a baseline of 18.46 to 13.35 at the 4-week follow-up and to 1.55 at the 52-week follow-up. These results confirm the substantial efficacy of dupilumab in improving both pruritus and quality of life for PN patients.
The NRS and DLQI scores showed more pronounced improvements in the early follow-up periods (4 weeks and 8 weeks), with subsequent, albeit less dramatic, improvements observed at the 52-week follow-up. This indicates that dupilumab has a notable impact on alleviating pruritus and enhancing quality of life in the initial treatment phase. Conversely, the IGA scores and nodule counts did not exhibit significant reduction during the first 16 weeks but demonstrated more pronounced improvement in later follow-up periods (16 weeks, 26 weeks, and 52 weeks). This suggests that dupilumab is more effective in reducing disease severity and nodule count with prolonged treatment. Thus, dupilumab is not only effective in the early stages of treatment but also maintains its therapeutic efficacy over the long term.
Beck et al. [29] reported in 2018 on 3 cases in the United States, demonstrating that within 12 weeks of treatment with dupilumab, there was a notable reduction in pruritus symptoms, a decrease in the overall size and number of prurigo lesions, and a marked improvement in quality of life. In a retrospective study conducted in Italy by Chiricozzi et al. [30] in 2020, significant improvement in skin lesions was first observed at 4 weeks, with further amelioration in pruritus and PN lesions evident by 36 weeks. Clinical improvement was assessed via the IGA, and both pruritus and insomnia, as measured by the NRS, showed substantial reductions.
A long-term retrospective study by Georgakopoulos et al. [31] in Canada in 2021 found that 78.9% of patients reported a subjective improvement in pruritus by 16 weeks, with 41.17% of patients demonstrating clinical symptom improvement by 52 weeks. In a 2023 real-world observational study conducted in China, Fang and Lian [32] observed that pruritus symptoms in PN patients exhibited rapid relief within 2 weeks of initiating treatment, with significant improvement in skin lesions noted at both 4 and 8 weeks. Additionally, Cao et al. [25] in a 2023 systematic review concluded that dupilumab treatment led to a significant reduction in NRS scores at weeks 4, 12, 16 and beyond 16 weeks, with at least 87.5% of PN patients achieving clinical remission. These findings are congruent with our prior clinical trial results.
Conversely, Cunha et al. [33] reported in 2022 from Portugal that a patient exhibited a decrease in NRS scores and a reduction in nodule count only after 3 months of dupilumab treatment. This suggests that adult patients with severe, treatment-resistant chronic PN, may require an extended period to achieve therapeutic response when treated with dupilumab.
Comparing the data from dupilumab treatment for atopic dermatitis [34–36] with for PN patients, it appears that dupilumab may require a longer duration of treatment to achieve its full therapeutic effects in PN. Unlike AD patients, those with PN experience a slower reduction in pruritus and a more gradual resolution of skin lesions.
In a comparison of our findings with a phase III open-label extension study by Blauvelt et al. [37], it is evident that the reduction in IGA scores was significantly greater in AD patients (1.5 points) compared to PN patients (0.19 points) at the 4-week mark. In terms of VAS scores, AD patients exhibited a more substantial decrease in pruritus, reaching 5.0, whereas PN patients had a lesser initial reduction of 1.41 points. Additionally, DLQI scores indicated that AD patients experienced a more significant improvement in quality of life (4.9 points) during the early treatment phase compared to PN patients (5.11 points).
However, in the later stages of treatment (16 weeks to 52 weeks), PN patients showed a more pronounced decrease in VAS scores, indicating more substantial relief from pruritus. Furthermore, the DLQI scores for PN patients decreased significantly in the later stages, reflecting a notable improvement in quality of life, while the improvement in AD patients was relatively gradual.
These differences in response times may be attributable to the distinct pathological characteristics and therapeutic response mechanisms of the diseases. AD patients may exhibit a more rapid initial response to dupilumab, but as treatment progresses, the rate of improvement may plateau. In contrast, PN patients may have a slower initial response, but with continued treatment, the therapeutic effects become more evident, demonstrating more significant long-term improvements, particularly in pruritus relief and quality of life enhancement.
In this research, adverse effects were generally mild and self-limiting, resolving either spontaneously or with symptomatic treatment. Notably, no cases of conjunctivitis – a commonly reported adverse reaction – were observed, and no patients discontinued therapy due to adverse effects, underscoring the favourable tolerability profile of dupilumab. Further investigation is warranted to characterize the nature of injection site reactions and determine their relationship with drug dosage or administration frequency. Additionally, the occurrence of facial erythema might be attributed to either local drug effects or individual skin sensitivity, necessitating further exploration of its underlying mechanisms.
In this research, limitations are conspicuous. We adopted the method of open design with no control and a pre-post self-comparison protocol was implemented. The enrolled patients had a history of poor response to conventional PN treatments. Since we hypothesized that the probability of clinical improvement without dupilumab was relatively low based on existing evidence and clinical experience, this self-comparison approach was selected. PN is a chronic, stubborn skin disease with patients suffering from severe itching and few treatment options. For patients open to biological agents, a placebo control might lead to early treatment dropout due to lack of expected results, causing ethical and practical issues. The study was in an outpatient setting. Patient communication about condition changes made a single-blind condition unfeasible. So, an open-label retrospective study was done. Despite its limitations like inability to confirm causal links as in a randomized trial and weaker evidence, it allows data collection for future in-depth studies, and provides valuable insights for future research under better conditions.
We allowed patients to use antihistamines and TCS, which might lead to potential biases in the final outcomes. Given that dupilumab takes time to exert its effects and there is no contraindication for concomitant medications, we did not compel patients to discontinue the use of antihistamines and TCS with less-than-ideal efficacy until the skin rashes and itching were truly alleviated.
While the infrequent and mild nature of adverse events supports the overall safety of dupilumab, the study’s limitations include a relatively small sample size and the potential overlap between atopic dermatitis and PN. Such overlap could impact treatment efficacy as patients with AD might exhibit a more pronounced initial response to dupilumab, potentially skewing the observed rates of clinical symptom relief. Future research should address these limitations by incorporating larger sample sizes and accounting for the effects of disease overlap on treatment outcomes.
The rates of observed clinical symptom relief may be affected by baseline patient traits, including disease duration, severity, and comorbid conditions. Thorough analyses of such baseline factors are crucial for pinpointing variables that notably influence treatment effectiveness. Moreover, patient compliance is crucial for assessing the long-term consequences of dupilumab treatment as it facilitates understanding the sustained efficacy of the drug in treating PN and ensures persistent adherence to the prescribed treatment protocol. These further analyses will provide a more comprehensive assessment of dupilumab’s efficacy, safety, and clinical relevance in the management of PN, thereby offering more robust evidence for its use in clinical practice.
Conclusions
Our data indicate that dupilumab is a highly effective and safe treatment option for PN. Dupilumab has shown significant benefits in reducing the number of prurigo nodules, improving IGA scores, alleviating pruritus, and enhancing the DLQI scores in patients with moderate to severe PN, while maintaining a favourable safety profile.
Compared to atopic dermatitis, patients with PN may require a longer duration of treatment with dupilumab to achieve comparable therapeutic effects. Although the emergence of various small-molecule drugs has expanded the treatment options for PN, these alternatives often present with greater limitations or side effects.
Currently, dupilumab stands out as a leading option for the treatment of PN due to its efficacy and safety. Future research should focus on conducting rigorous randomized controlled trials to further validate these findings. Such studies will facilitate the optimization of treatment regimens and the development of personalized therapeutic strategies tailored to individual patient profiles, ultimately improving outcomes and quality of life for PN patients.